2021 Volume 62 Issue 12 Pages 1739-1744
2021 Volume 62 Issue 12 Pages 1739-1744
Organic-inorganic dual-coated photocatalytic TiO2 nanoparticles (NPs) were obtained by two-step surface modification of i) an inner SiO2-coating and ii) outer alkylation through a silane coupling reaction, exhibiting tunable photocatalytic activity. Evaluation of the photocatalytic activity under ultraviolet ray irradiation revealed that the dual coating on the surface of the TiO2 NPs changed the reaction rate-determining step of the photocatalytic decomposition of methylene blue (MB) from an adsorption-limited manner to a diffusion-limited manner. Unmodified TiO2 NPs decomposed MB as soon as MB was adsorbed on the surface, while the decomposition reaction proceeded in the concentration-independent diffusion-controlled manner in the surface-modified NPs. The double shells might confine both outward excited electron diffusion and inward substance diffusion, preventing the generation of active oxygen on the surface of TiO2 NPs.

Recent advances in polymer synthesis technology1–8) have made polymers such as resins and plastics widely used to replace metal and structural materials9) due to their light weight properties, high mechanical strength, and easy malleability.2) However, the use of polymers under light causes degradation responsible for yellow discoloration and cracking.10,11) Ultraviolet (UV) absorbers such as benzotriazole,12,13) benzimidazole,14) and benzophenone15,16) are added into polymers to improve light stability; however, such organic UV absorbers are also gradually decomposed by light irradiation. In the field of inorganic chemistry, semiconductor materials and photocatalysts are representatives of light absorbing materials17,18) and various photocatalysts for organic matter decomposition and water splitting have been developed.19,20) Titanium oxide (TiO2) nanoparticles (NPs) are well-known photocatalysts with high UV absorbing abilities and they have been put to practical use in various fields.21,22) TiO2 NPs are also used as UV scattering agents for polymer-stabilization23,24) due to their high refractive indices. These inorganic UV stabilizers are highly durable even under UV light irradiation. However, TiO2 NPs generate active oxygen species by light irradiation thus decomposing the polymer. Here, photocatalytic degradation occurs only on the interfaces between polymers and TiO2 NPs. Therefore, suppressing the surface reaction leads to the utilization of photocatalytic NPs as new UV absorbers with high durability for polymer stabilization. There have been some reports that the reaction rate of the photocatalyst is slowed by SiO2- and Al2O3-coating.25–28) In this study, we have developed organic-inorganic dual-coated TiO2 NP photocatalysts and investigated their photocatalytic activity. Figure 1 exhibits a schematic illustration of the dual-coated TiO2 NP. The TiO2 NP is doubly modified with an inner inorganic SiO2 shell and an outer organic alkyl mantle. The inner SiO2 shell is designed to limit diffusion of excited electrons and holes to the surface while the outer alkyl mantle shuts out substance diffusion of O2 and H2O molecules from the NP’s surroundings.

A model of the organic-inorganic dual coating of TiO2 NPs and confinement of both outward and inward diffusions.
All reagents of the highest commercial quality and solvents (Aldrich Chemicals, FUJIFILM Wako Pure Chemicals, Nacalai Tesque, or Ishihara Sangyo Kaisya) were used as received. Water was doubly distilled and deionized prior to use.
Transmission electron microscopic (TEM) images were obtained using a JEOL JEM-2100 with an acceleration voltage of 200 kV. Thermogravimetric analysis (TG) was performed using an SII TG/DTA6300. UV visible absorption spectroscopy (UV-vis) diffuse reflectance spectra were obtained using a JASCO V-660 with an integrating sphere unit. Photocatalytic activity test was carried out using a circulating photolysis consisting of an ultraviolet water sterilizer UV-1011 (Shann Chih Enterprise Co., Ltd.) with a 6 W germicidal lamp (wavelength of 253.7 nm) and a perista pump (equipment details in Supporting Information).
2.2 Nanoparticles synthesisFirst, 0.1 g of TiO2 NPs (ST21, Ishihara Sangyo Kaisha Ltd.) and 0.5 g of polyvinylpyrrolidone (PVP) were dispersed into 80 mL of ethanol and sonicated for 4 h in the dark. After removing excess PVP by centrifugation, the resultant precipitate was re-dispersed into 60 mL of ethanol. Then, 1.7 mL of aqueous ammonia (28%) and tetraethylorthosilicate (TEOS) were added into the mixture. It was stirred at room temperature in the dark for 24 h. The amount of TEOS was changed to 0.5 mL and 1.3 mL, respectively, so that the SiO2 thickness was adjusted to 3 nm and 6 nm, obtaining SiO2-coated TiO2 NPs TS3 and TS6. The resulting NPs were purified by centrifugation (15,000 rpm) with ethanol three times. Further alkyl coating was applied to the TS3 and TS6 via a silane coupling reaction using dodecyltrimethoxysilane. SiO2-coated TiO2 NPs (0.1 g) were dispersed into 10 mL of toluene and 2.0 mL of oleylamine were added into the mixture. After ultrasonication, 0.1 mL of dodecyltrimethoxysilane and 20 µL of ion-exchanged water were added into the mixture. The mixture was stirred at 100°C for 24 h. The resulting product was centrifuged (15,000 rpm) with ethanol three times to give TS3C12 and TS6C12 from TS3 and TS6, respectively.
Figure 2 shows TEM images of untreated ST21, TS3, and TS6. ST21 was elliptical in shape and approximately 20 nm in size. Figure 2(b) shows that high contrast particles are encircled by low contrast layers with 3 nm thickness. Magnified images revealed that high contrast particles were assigned to be TiO2 from the lattice fringe of the (112) plane of anatase-type TiO2. The XRD pattern of ST21 shown in Fig. S1 also revealed that ST21 consists of anatase type of TiO2 phase. All particles were covered with a uniform SiO2 layer regardless of the amount of TEOS. TEM observation and TG measurements revealed that the volume fractions of SiO2 to TiO2 were calculated as 54.4% and 75.6%, while the weight fractions of SiO2 to TiO2 were 38.4% and 61.7% for TS3 and TS6, respectively. The amount of alkyl modification per particle was 12,000 and 14,000 for TS3C12 and TS6C12, respectively. The detailed calculation is summarized in SI (Fig. S2).

TEM images of SiO2 coated TiO2 NPs: (a) unmodified TiO2 NPs: ST21; (b) TS3 with a SiO2 shell (thickness: 3 nm); (c) TS6 with a SiO2 shell (thickness: 6 nm); (d) Magnified image of (b). The scale bar in (c) is common for (a) and (b).
Figure 3 shows the absorption spectra transformed from the powder diffuse reflectance spectra of the SiO2-coated TiO2 NPs by the Kubelka-Munk formula. TS3 and TS6 absorbed UV light below 380 nm as well as untreated ST21 in spite of thick silica layers. It is clear from the graph that the SiO2 coating does not interfere with the UV absorption of TiO2 NPs.

Absorption transformed from the powder diffuse reflectance spectrum of the SiO2 coated TiO2 NPs by the Kubelka-Munk formula.
Photocatalytic activity of the SiO2-coated TiO2 NPs was evaluated by the decomposition reaction of methylene blue (MB) under UV light irradiation using a circulation type photolysis apparatus as shown in Fig. S3. The SiO2-coated TiO2 NPs were dispersed into ion-exchanged water and the concentration of substantive TiO2 NPs was adjusted to 50 mg/L. MB was added to the dispersions at a concentration of 5.0 mg/L. The dispersions were circulated at a flow rate of 115 or 240 mL/min by a perista pump for 80 minutes under UV light irradiation with a wavelength of 253.7 nm, and time-dependent changes in the absorbance of MB were measured. Absorbance was measured after collecting 10 mL of solution and removing particles by centrifugation. To clarify the effect of MB adsorption onto the particle surface, the TS3 dispersion was circulated for 80 minutes without UV light irradiation. Figure 4(a) shows the result when the flow rate was 240 mL/min. In this case, most MB was decomposed after 80 minutes with ST21. In contrast, the final MB decrements were reduced to 58.1% and 25.8% after 80 minutes with TS3 and TS6, respectively. The amount of MB adsorbed to the surface of the NPs was estimated to be 0.4% from TS3 without UV irradiation. Similar results have been reported elsewhere.25,26) From the outline of the graphs, we see that MB decreased in a concentration-dependent manner in the case of ST21, whereas in the cases of TS3 and TS6, MB linearly decreased from the UV light irradiation regardless of the MB concentrations. This indicates that MB decomposition by ST21 is an adsorption limited reaction in which MB decomposed immediately when adsorbed on the particle surface. On the other hand, MB decomposition by TS3 and TS6 proceeded in a diffusion limited manner dominated by the mass diffusion in the amorphous SiO2 layer. The reaction rate constants were calculated as −6.10 × 10−4, −7.50 × 10−5, and −4.72 × 10−5 s−1 for ST21, TS3, and TS6, respectively, and the photocatalytic reaction was controlled with a shell thickness of SiO2 coating on the TiO2 NPs (see Fig. S4(a)).

Time-dependent MB concentrations of the SiO2-coated TiO2 NPs. The flow rates were (a) 240 mL/min and (b) 115 mL/min.
MB almost fully decomposed in 80 minutes even with SiO2-coated particles when the flow rate was as low as 115 mL/min (Fig. 4(b)). The reason why the photodegradation rate of the SiO2-coated NPs has changed will be discussed later. According to the results of a UV nonirradiation test, there was a slight increase in the amount of MB reduction that is considered to be derived from adsorption. Since the MB concentration decreased in a concentration-dependent manner, we can conclude that the degradation progressed in an adsorption-limited manner. This is due to the increase in the supply of active oxygen from the slow flow rate and prolonged exposure to UV light. The reaction rate constants of MB decomposition excluding the adsorbed amount were calculated as 7.59 × 10−4, 6.73 × 10−4, and 6.81 × 10−4 s−1 for ST21, TS3 and TS6, respectively (see Fig. S4(b)). Therefore, the thick SiO2 layer on the surface of TiO2 NPs slowed the mass diffusion and reduced the photocatalytic activity.
Since the alkyl-modified TiO2 NPs were not dispersed in water, their photocatalytic activities were evaluated by MB decomposition in organic solvents. The alkyl/SiO2 dual-coated TiO2 NPs were dispersed into hexane/ethanol (9/1 v/v) and the concentration of substantive TiO2 NPs was also adjusted to 50 mg/L. This mixed solvent assumed some proportion of hydrophilic functional groups in the resin. MB concentration, UV wavelength, and circulation time were the same conditions as above. The flow rate was set to 115 mL/min. Here, time-dependent MB absorbance was measured after collecting 10 mL of the hexane/ethanol solution, then adding 10 mL of ion-exchanged water and extracting MB completely into the aqueous phase. Despite a flow rate of 115 mL/min and 80 minutes of UV light irradiation, 20.2% and 49.3% of MB remained for TS3C12 and TS6C12, respectively, shown in Fig. 5. The adsorption amount was estimated as 14.4% from a fitted curve, so that the actual amounts of decomposed MB were estimated as 65.4% and 34.9% for TS3C12 and TS6C12, respectively. Therefore, the photocatalytic reaction rates were reduced by 76.4% and 42.4%, respectively, compared to before the alkyl modification. Here, the TG measurement of the TS6C12 after the reaction revealed that 10.4% of alkyl ligands on the surface of TS6C12 was decomposed during the photocatalytic activity test (see, Fig. S5). The weights of the decomposed ligand and MB in 1 L of the reaction solution were 0.65 and 1.75 mg, respectively, indicating that MB has a faster decomposition rate than that of ligands. The decrease in reaction rate at a slow flow rate is likely due to the formation of an alkyl chain layer suppressing the material diffusion in the silica layer. In fact, from the graph, we see the somewhat adsorption-limited MB decomposition with TS3C12, while MB decomposed in the diffusion-limited manner with TS6C12. Here, the reaction rate constants were calculated to be −3.24 × 10−4 and −6.16 × 10−5 s−1 for TS3C12 in the adsorption-limited manner and TS6C12 in the diffusion-limited manner, respectively (see Fig. S4(c)). The reaction rates decreased despite the low flow rate, presumably because the formation of the alkyl mantle suppressed the mass diffusion into the SiO2 layer. This suggests that photocatalysis is further suppressed by the formation of an alkyl layer. In addition, the increase in the MB adsorption amount on particles indicates that the affinity for resin and polymer has been significantly improved, leading to a potential application as a new UV absorber for polymers and resins. Since the material diffusion rate in polymers is considered to be extremely slow, these resulting dual-coated NPs have great potential as a new type of UV absorber.

Time-dependent MB concentrations of the alkyl/SiO2 dual-coated TiO2 NPs. The flow rate was 115 mL/min.
In conclusion, organic-inorganic dual-coated TiO2 NPs have been developed by a two-step silica coating and alkyl coating process on TiO2 NPs, and their photocatalytic activity in an organic solvent under UV irradiation was evaluated. Compared to unmodified TiO2 NPs, the resulting NPs show a reduced photocatalytic reaction rate, controlling outward and inward diffusion with dual layers, and a good affinity for polymers and resins. Such dual-coated NPs have great potential as a new type of organic-inorganic UV absorber with high light absorbing properties and high durability for various polymers. These properties can be attributed to an extremely slow material diffusion rate in polymers.
This work was performed under the Research Program “Dynamic Alliance for Open Innovation Bridging Human, Environment and Materials” in the “Network Joint Research Center for Materials and Devices” and the Cooperative Research Program of “Network Joint Research Center for Materials and Devices”. This work was partly supported by the “KOSEN 4.0” Initiatives.
Figure S1 shows an XRD pattern of ST21, revealing that ST21 consists of anatase type of TiO2 phase.
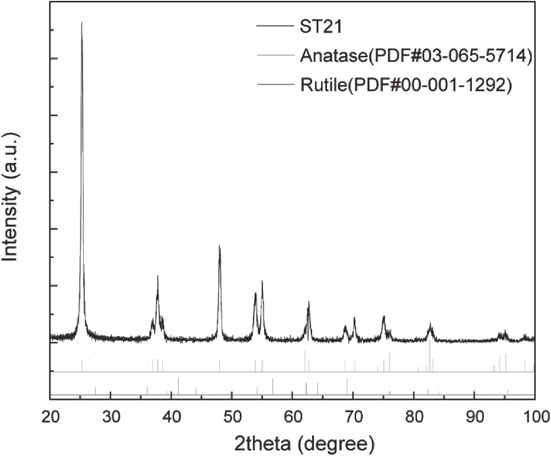
An XRD pattern of ST21 used as TiO2 NPs in this study.
TG measurement was performed at a heating rate of 10°C/min in air. Figure S2 shows TG charts of organic-inorganic dual coated TiO2 NPs TS3C12 and TS6C12. 13.2% and 12.5% of organic components for TS3C12 and TS6C12 were burned off, respectively.

TG charts of organic-inorganic dual-coated TiO2 NPs TS3C12 and TS6C12.
The volume of SiO2 layer of one TS3 NP (VSiO2,TS3) was calculated by the following equation: VSiO2,TS3 = 4π(DTS3 × 10−7/2)3/3 − 4π(DST21 × 10−7/2)3/3. Here, DTS3 and DST21 are the particle mean size of TS3 and ST21 determined by TEM as shown in Fig. 2(a) and 2(b). As a result, VSiO2,TS3 of TS3 was 5.0 × 10−18 cm3. Following this, the weight of SiO2 layer for one TS3 NP (WSiO2,TS3) was 1.1 × 10−17 g calculated from WSiO2,TS3 = VSiO2,TS3 × ρSiO2. The density of amorphous SiO2 ρSiO2 is 2.2 g cm−3. On the other hand, VST21 and WST21 were 4.2 × 10−18 cm3 and 1.8 × 10−17 g using the density of TiO2 (ρTiO2) as 4.23 g cm−3. Moreover, VSiO2,TS6 and WSiO2,TS6 were also calculated as 1.3 × 10−17 cm3 and 2.9 × 10−17 g. From the above results of TG measurements, the weight losses of TS3C12 and TS6C12 were 13.2% and 12.5%, respectively. Thus, the ligand amount of TS3C12 (NL,TS3C12) was 12,000 calculated as NL,TS3C12 = (WSiO2,TS3 + WST21) × 0.132/(1 − 0.132)/MWC12H25/NA. Here, avogadro number NA is 6.022 × 1023 and MWC12H25 is 169.32. The similar calculation revealed that NL,TS6C12 was 14,000.
4. Photocatalytic activity evaluation by MB decompositionThe photocatalytic activity was evaluated by MB decomposition reaction under irradiation of UV light using a circulation type photolysis apparatus as shown in Fig. S3. 1 L of SiO2-coated TiO2 NPs dispersion with 50 mg/L of TiO2 concentration was prepared and 5.0 mg/L MB was mixed into the solution. It was circulated for 80 min with flow rate of 240 or 115 mL/min under UV irradiation (wavelength of 253.7 nm) in the reaction stainless tube. The UV sterilizer is a tube shape with a diameter of 50 mm and a height of 230 mm, and a germicidal lamp covered with a 23 mm diameter quartz glass inside. Thus, residence times were estimated as 92 and 190 s for the flow rates of 240 and 115 mL/min, respectively. 10 ml of the dispersions were collected in every 10 minutes, and absorbance was measured after being centrifuged to remove NPs.

A schematic illustration of the equipment configuration.
Organic-inorganic dual-coated TiO2 NPs were dispersed into hexane/ethanol (9/1 v/v). MB concentration, UV wavelength and circulation time were the same conditions as above. The flow rate was set to 115 mL/min. 10 mL of dispersions were also collected in every 10 minutes, and MB was extracted with 10 mL of water to remove NPs. The resulting 10 mL of aqueous MB solution was provided to absorbance measurement.
5. Calculation of reaction rateAssuming that the decrease in MB absorbance follows the first-order reaction rate constant, the reaction rate constant was determined as following formula (eq. (A-1)). C: MB absorbance, C0: MB initial absorbance, k: first-order reaction rate constant, t: time.
| \begin{equation} \ln\left(\frac{C}{C_{0}}\right) = -kt \end{equation} | (A-1) |
| \begin{equation} C - C_{0} = -k't \end{equation} | (A-2) |

MB decomposition rates. (a) TS3 and TS6 calculated from eq. (A-2) and ST21 calculated from eq. (A-1) in the flow rate of 240 mL/min. (b) TS3, TS6 and ST21 calculated from eq. (A-1) in the flow rate of 115 mL/min. (c) TS3C12 calculated from eq. (A-1) and TS6C12 calculated from eq. (A-2) in the flow rate of 115 mL/min.
Figure S5 shows TG charts of TS6C12 before and after the photocatalytic activity test. In the case of TS6C12, the value of the organic ligands ratio on the surface of the photocatalyst NPs decreased to 11.2% from 12.5% after the photocatalytic activity evaluation, indicating that the 10.4% of the alkyl ligands was also decomposed by the photocatalytic activity test. Here, the weight of TiO2 NPs in 1 L of the reaction solution was 50 mg, so that the weight of the decomposed alkyl ligands on the surface of NPs was estimated to 0.65 mg, while 1.75 mg of MB in 1 L of solution, being calculated from the value of 34.9%, was decomposed during the photocatalytic activity test. Therefore, the decomposition rate of MB is considered to be 2.7 times faster than that of the alkyl ligands in our system.

TG charts of TS6C12 before and after photocatalytic activity test.