2021 Volume 62 Issue 5 Pages 655-660
2021 Volume 62 Issue 5 Pages 655-660
The relationship between chloride ion concentration and pH on de-passivation of steel rebar embedded in concrete was investigated by a two frequencies impedance measurement using probe electrodes. A saturated calcium hydroxide solution was used for the electrolyte solution as a simulated concrete pore solution. The pair of probe electrodes, which simulates the steel rebar, was immersed in the electrolyte solution prepared by an arbitrary concentration of chloride ions, and the two frequencies impedance measurement was performed. Air was supplied to the electrolyte solution to simulate the neutralization of concrete during the measurement. The charge transfer resistance related to the electrode/solution interface, Rct, was estimated from the two frequencies impedance measurement of probe electrodes in each electrolyte solution. This demonstrated that the Rct was drastically reduced by decreasing the pH of the electrolyte solution, namely, the de-passivation of steel rebar. The corrosion conditions of steel rebar were categorized as “passive state,” “de-passivation,” and “corrosion” based on the changes in the monitoring of Rct and pH in each electrolyte solution. These results were plotted on the two-dimensional diagram composed of chloride ion concentration and pH of the electrolyte solution. The conditions for the de-passivation of the passive film on steel rebar were discussed.
This Paper was Originally Published in Japanese in Zairyo-to-Kankyo 68 (2019) 303–308.

Fig. 2 Schematic of probe electrodes in the electrolyte solution and equivalent circuit between two electrodes. The Rct is the charge transfer resistance at the electrode/solution interface, the Rsol is the solution resistance, and the Cdl is electric double-layer capacitance at the electrode/solution interface.
Reinforced concrete is often used for infrastructures such as expressways because of its high durability. However, the reduced durability of concrete related to the corrosion of steel rebar in concrete becomes a serious problem. The steel rebar/concrete interface in reinforced concrete has porous structures with high porosity, called transition zone.1) Because the calcium hydroxide, which is the cement component, is dissolved into the solution within transition zone1) from concrete, the solution in the transition zone can keep at high pH (around pH = 12). Thus, the passive film can be formed on the steel rebar in concrete. Therefore, the steel rebar in concrete shows high corrosion resistance. When the carbon dioxide penetrates the concrete from the air, the pH of electrolyte solution inside concrete slightly decreases around pH = 10 due to changes of calcium hydroxide to calcium carbonate. This phenomenon is known as neutralization and causes the corrosion of steel rebar in concrete. Moreover, the corrosion of steel rebar occurs due to the de-passivation of passive film formed on the steel rebar in concrete when the chloride ions penetrate inside the concrete and reach near the steel rebar. This phenomenon is known as salt damage.
Regarding the occurrence of steel rebar corrosion related to the neutralization and salt damage, some researchers have reported the effect of pH and chloride ions on the occurrence of corrosion of steel rebar in concrete. Whitman et al.2) investigated the corrosion rate of iron from the amount of dissolved oxygen loss in electrolyte solution at arbitrary pH. They2) reported that the corrosion rate of iron decreased owing to the film formation on the surface when the pH of the electrolyte solution was above 10. Hausman3) investigated the conditions of occurrence of steel rebar corrosion based on the relation between chloride ion concentration and hydroxide ion concentration in the pore solution. In this report,3) each available atom of iron not already in the protective oxide film may be considered the object of a game of chance between hydroxyl and chloride ions.
Furthermore, the threshold of [Cl−]/[OH−] = 0.6 is proposed based on the calculation of the probability of events. Therefore, the dissolution rate of steel rebar is increased when the ratio [Cl−]/[OH−] is larger than 0.6. Hausman3) performed the rest potential measurement of mild steel in the electrolyte solution, which simulates the concrete environment containing chloride ions. In this measurement, the range of pH is 11.6 to 13.2. Hausman3) confirmed that the corrosion conditions of mild steel evaluated by their measurements are in good agreement with that estimated from the ratio [Cl−]/[OH−].
As mentioned above, the occurrence of steel rebar corrosion is affected by pH and chloride ion concentration in concrete. However, it is vital to monitor the process of the passive state, de-passivation, and corrosion of steel rebar on the salt damage and neutralization to accurately obtain the conditions of steel rebar in concrete.
Our group4,5) have developed a probe electrode for corrosion monitoring of steel rebar embedded in concrete. A pair of probe electrodes is embedded near steel rebar in concrete during this measurement, and a two frequencies impedance measurement performed to obtain charge transfer resistance.4,5) In this study, the two frequencies impedance measurement of a pair of probe electrodes was implemented in a saturated calcium hydroxide solution, which simulates a concrete pore environment. The air was pumped to the electrolyte solution containing an arbitrary concentration of chloride ions during measurement to simulate the neutralization and salt damage of concrete. The effect of chloride ions concentration and pH on de-passivation of passive film formed on steel rebar in concrete was discussed based on the results of the monitoring of Rct obtained by the two frequencies impedance measurement and pH of an electrolyte solution.
In this study, two frequencies impedance measurement of probe electrodes were performed in the saturated calcium hydroxide solution. It contained an arbitrary concentration of chloride ions for corrosion environment investigation on reinforced concrete structures using probe electrodes. Figure 1(a) shows a schematic of the probe electrode. Details of the development of probe electrodes were previously described.4,5) A deformed reinforcing bar (JIS standard: SD345, C: lower than 0.27%, S: lower than 0.040%, C + Mn: lower than 0.50%) was used as the electrode with a diameter and thickness of 1.0 cm and 0.8 cm, respectively. The top and bottom surface of the electrode were insulated by epoxy resin.
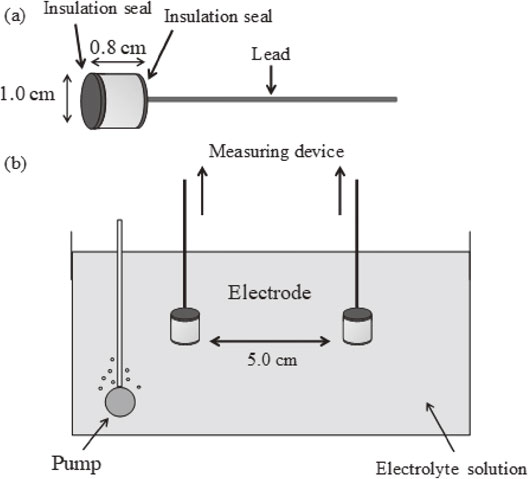
Schematic of (a) probe electrode and (b) measurement system.
Approximately 15 saturated hydroxide solutions containing an arbitrary concentration of chloride ions were used for the electrolyte solution. In this case, the concentrations of chloride ions in these solutions were in the range of 0 mmol/dm3 to 34 mmol/dm3. Figure 1(b) presents a schematic of impedance measurement using probe electrodes. A pair of probe electrodes with an electrode spacing of 5.0 cm was immersed in the electrolyte solution. A two-electrode system performed the two frequencies impedance measurement of the pair of probe electrodes. Figure 2 represents the schematic of probe electrodes in the electrolyte solution and the equivalent circuit between two electrodes. In the equivalent circuit of Fig. 2, the Rct is the charge transfer resistance at the electrode/solution interface, the Rsol is the solution resistance, and the Cdl is electric double-layer capacitance at the electrode/solution interface. As previously reported,4,5) the two frequencies impedance measurement was performed using the same equipment (CEM-100A). The impedance at 10 kHz and 10 mHz was automatically measured every 10 min. In this measurement, the real part of impedance at 10 kHz (Z′10 kHz), the real part of impedance at 10 mHz (Z′10 mHz) and the imaginary part of impedance at 10 mHz (Z′′10 mHz) were used for the calculation of Rct. The Rct was calculated by eq. (1), as proposed by Haruyama et al.:6,7)
| \begin{equation} R_{\text{ct}} = (Z'{}_{\text{10mHz}} - Z'{}_{\text{10kHz}} + (Z''{}_{\text{10mHz}}{}^{2}/(Z'{}_{\text{10mHz}} - Z'{}_{\text{10kHz}})) \end{equation} | (1) |
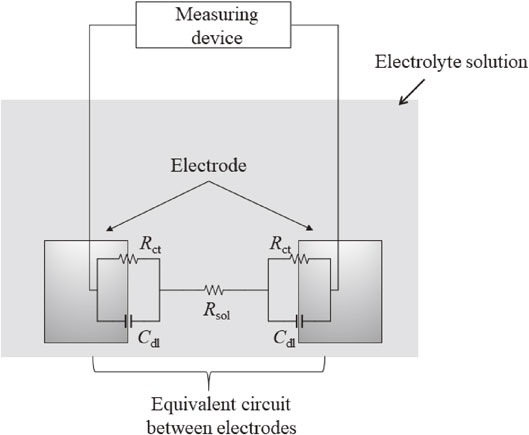
Schematic of probe electrodes in the electrolyte solution and equivalent circuit between two electrodes. The Rct is the charge transfer resistance at the electrode/solution interface, the Rsol is the solution resistance, and the Cdl is electric double-layer capacitance at the electrode/solution interface.
In the two frequencies impedance measurement, an AC amplitude was 10 mV, and an applied DC voltage was 0 V. The air was pumped into the electrolyte solution using a pump during measurement to simulate the neutralization of concrete. The pH of the electrolyte solution was measured every 24 h to investigate the de-passivation of passive film formed on the electrodes. The pH measurement was conducted by inserting the pH electrode near the electrode in the electrolyte solution. The pH meter (DKK-TOA CORPORATION, HM-30G) was used for the pH measurement.
2.2 Measurement of the impedance spectrumA two-electrode system measured the impedance spectrum of probe electrodes to confirm the values of Rct estimated by the two frequencies impedance measurement of probe electrodes. A potentio/galvanostat (SI1287, Solartron) and a frequency response analyzer (SI1255B, Solartron) were used for the measurement. The measurements were performed in the frequency range of 10 mHz to 1 kHz at five frequencies per decade, an AC amplitude of 10 mV, and an applied DC voltage of 0 V.
Figure 3 shows changes of Rct estimated from two frequencies impedance measurement of probe electrodes and pH of the electrolyte solution. The concentration of chloride ions in the electrolyte solution is 1.7 mmol/dm3. In Fig. 3, the Rct is increased after starting the measurement and becomes approximately 1.0 MΩ after 30 h. The Rct then increases and decreases in the range of 0.5 MΩ to 1.4 MΩ and is drastically decreased after 221 h. The pH of the electrolyte solution slightly decreased over time and reached 11.65 before the rapid decrease of Rct at 216 h. The pH of the electrolyte solution was at 11.14 after the rapid decrease of Rct at 240 h, and the pH drastically decreased over time. Tokieda4) et al. conducted the two frequencies impedance measurement in saturated calcium hydroxide solution (pH = 12). They indicated that the increase of Rct after the measurement is related to the formation and growth of passive film on the electrodes. Furthermore, the rapid decrease of Rct observed around pH = 10.5 is associated with the de-passivation of the passive film on the electrodes based on the results of Rct estimated from the eq. (1) and pH measurement.4)
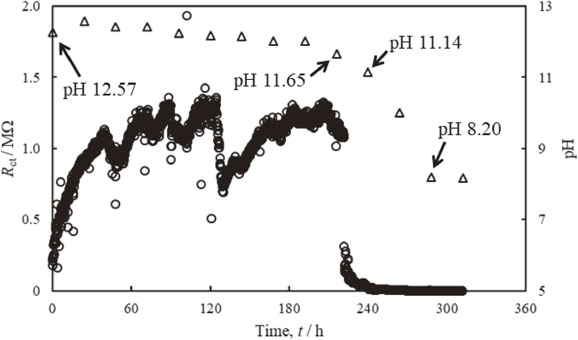
Changes of Rct estimated from two frequencies impedance measurement of probe electrodes and pH of an electrolyte solution. The concentration of chloride ions in the electrolyte solution is 1.7 mmol/dm3.
In contrast, it is considered that the increase of Rct after starting measurement indicates the formation and growth of passive film, as seen in Fig. 3. This is due to the high pH and competition reaction related to the formation and dissolution of the passive film that occurs owing to the presence of chloride ions in the electrolyte solution. Thus, the repeated increase and decrease of Rct is observed in Fig. 3. In addition, the rapid decrease of Rct corresponded to a decrease of pH between 216 h and 240 h is related to the dissolution or de-passivation of passive film.4) The Rct shows small values after de-passivation of the passive film on the electrodes, indicating the progress of the dissolution of the electrode.
The measurement of the impedance spectrum of pair of probe electrodes was performed before and after the de-passivation of the passive film on the electrodes to confirm the values of Rct estimated by the two frequencies impedance measurement.
Figure 4(a) presents the impedance spectrum of a pair of probe electrodes after 216 h. A part of the loop is observed in the impedance spectrum in Fig. 4(a). The Rct is estimated from the diameter of the loop by extrapolating the part of the loop, demonstrating that the Rct is approximately 2.80 MΩ. Figure 4(b) represents the impedance spectrum of the pair of probe electrodes after 240 h. The Rct is estimated to be 0.0103 MΩ from the diameter of the loop by extrapolating the part of the loop in Fig. 4(b). These results indicate that the order of Rct after 216 and 240 h in Fig. 4(a) and Fig. 4(b) is in good agreement with that in Fig. 3. It is confirmed that the two frequencies impedance measurement of pair of probe electrodes can be applied to the monitoring of Rct in the present conditions.
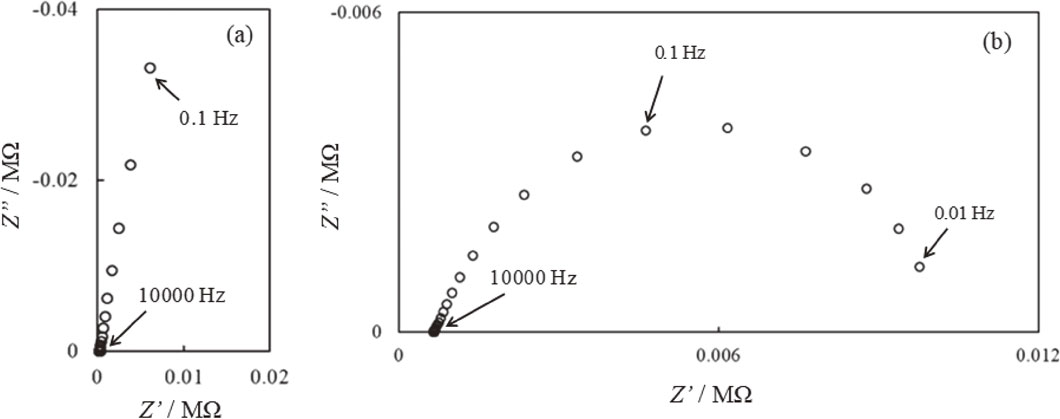
Impedance spectra of probe electrodes in saturated calcium hydroxide containing 1.7 mmol/dm3 NaCl after (a) 216 h immersion and (b) 240 h immersion.
The monitoring of Rct and pH was conducted by measuring two frequencies impedance of probe electrodes and pH near the electrode in saturated calcium hydroxide solution with different concentrations of chloride ions.
Figure 5 shows Rct estimated by two frequencies impedance measurement of probe electrodes and pH near the electrode in saturated calcium hydroxide solution with different concentrations of chloride ions. The concentrations of chloride ions were 0.9 mmol/dm3, 3.4 mmol/dm3 and 5.1 mmol/dm3. In Fig. 5(a), the Rct is increased just after starting impedance measurement. The repeated increase and decrease of Rct is observed after 20 h. The Rct is decreased rapidly around 181 h, and Rct then shows small values. The pH is slightly decreased just after starting measurement and drastically decreased between 168 and 192 h. Though the increase of Rct is observed just after starting measurement in the concentration of chloride ions of 3.4 mmol/dm3 presented in Fig. 5(b), the Rct is drastically decreased and then increased again around 49 and 70 h. The Rct is decreased rapidly around 168 h and Rct then indicates small values.
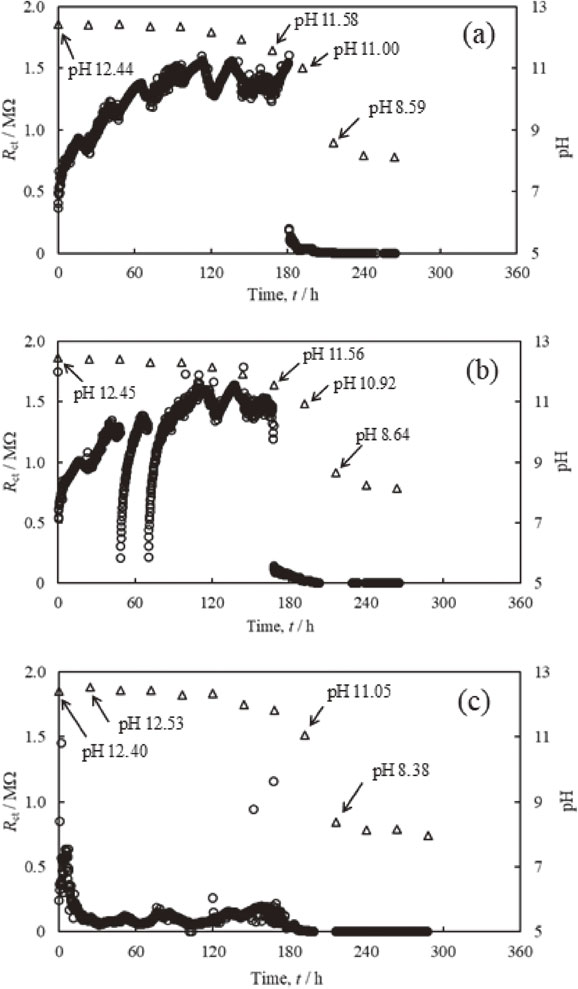
Changes of Rct estimated from two frequencies impedance measurement of probe electrodes and pH of an electrolyte solution. The concentration of chloride ions in electrolyte solution is (a) 0.9 mmol/dm3, (b) 3.4 mmol/dm3 and (c) 5.1 mmol/dm3.
Furthermore, the pH is decreased gently shortly after starting measurement and drastically decreased between 168 and 216 h. In the case of the concentration of chloride ions of 5.1 mmol/dm3, as shown in Fig. 5(c), the increase of Rct is observed shortly after starting measurement and then decreased rapidly. The pH is slightly decreased shortly after starting measurement and then drastically decreased between 168 and 216 h.
We determined the pH at a rapid decrease of Rct observed in Figs. 5(a) and 5(b), as the pH at de-passivation of steel rebar. Although it is important to measure the pH near the electrode whose surface is just after de-passivation, it is difficult to predict the event of de-passivation during measurement. In this study, the pH at de-passivation of the passive film on the electrode was estimated by assuming the pH changed linearly with time before and after de-passivation. Accordingly, the pH at the de-passivation of the passive film on the electrode in Figs. 5(a) and 5(b) was estimated to be 11.16 and 11.56, respectively. These results demonstrate that the pH at the de-passivation of the passive film on the electrode is increased by increasing the concentrations of chloride ions. In the case of chloride ion concentration of 5.1 mmol/dm3, as shown in Fig. 5(c), the pH shows a high value until 168 h. However, 13 h later, the Rct becomes approximately 0.2 MΩ. Though the film formation is performed on the steel rebar because of the high pH of electrolyte solution from shortly after stating measurement, the dissolution of steel rebar related to the de-passivation of steel rebar caused by the increase of chloride ion concentration is superior to the film formation on the steel rebar. The dissolution and formation are repeated shortly after starting measurement in the chloride ion concentration of 0.9 mmol/dm3 (Fig. 5(a)) and 3.4 mmol/dm3 (Fig. 5(b)). However, the pH associated with the de-passivation of steel rebar becomes high due to the increase of the chloride ion concentration. Further, it is implied that the dissolution of steel rebar due to the de-passivation occurs shortly after stating measurement in the case of the relatively high chloride ion concentration like 5.1 mmol/dm3 [Fig. 5(c)].
3.3 Relationship between chloride ions concentration and pH on corrosion conditions of steel rebar in concreteIn sections 3.1 and 3.2, the conditions of steel rebar could be categorized into three categories, namely (i) passive state, (ii) de-passivation, and (iii) corrosion. This is concluded by the monitoring of Rct estimated from impedance measurement of probe electrodes and pH near the electrode in saturated calcium hydroxide solution with an arbitrary concentration of chloride ions. The relationship between chloride ion concentrations and pH on the conditions of steel rebar in concrete were discussed in this section.
Figure 6 represents the relationship between chloride ion concentrations and pH on the conditions of steel rebar in concrete. The outcome is based on the results of monitoring of Rct estimated from impedance measurement of probe electrodes and pH near the electrode in saturated calcium hydroxide solution with an arbitrary concentration of chloride ions. The vertical axis and horizontal axis are the chloride ion concentrations of the electrolyte solution used in the measurement and pH near the electrode during the measurement, respectively. As the chloride ion concentrations of electrolyte solution are expressed by logarithmic in the figure, the value of 0 mmol/dm3 is approximately inserted in the axis. The upper horizontal axis and lower horizontal axis denote pH and hydroxide ion concentrations, respectively. By determining the event of de-passivation of steel rebar from the monitoring of Rct that estimated from impedance measurement of probe electrodes and pH near the electrode, we defined before de-passivation, an event of de-passivation and after de-passivation as “passive state,” “de-passivation” and “corrosion,” respectively. If the Rct indicates a minimal value from just after starting the measurement, as shown in Fig. 5(c), the condition of steel rebar was defined as “corrosion.” The △, ○ and ▲ correspond to “passive state,” “de-passivation,” and “corrosion” of steel rebar, respectively. Whitman et al.2) reported that the corrosion rate of steel rebar is decreased at pH above 10. When the pH is below 10, the condition of steel rebar is corrosion (▲) in Fig. 6. This result is in agreement with the literature reported by Whitman et al.2) Further, Hausman3) suggested that the relationship between chloride ion concentration [Cl−] and hydroxide concentration [OH−] on de-passivation of steel rebar surface can be expressed by [Cl−]/[OH−] = 0.6. Focusing on the dotted line ([Cl−]/[OH−] = 0.6) and plots obtained from the present study in Fig. 6, the plots do not obey the relation [Cl−]/[OH−] = 0.6 when the chloride ion concentration is larger than 3.4 mmol/dm3. Moreover, in the case of chloride ion concentration larger than 5.1 mmol/dm3, the condition of steel rebar is corrosion (▲) regardless of pH in Fig. 6. This indicates that the de-passivation of steel rebar occurs in the high concentration of chloride ions regardless of pH values.
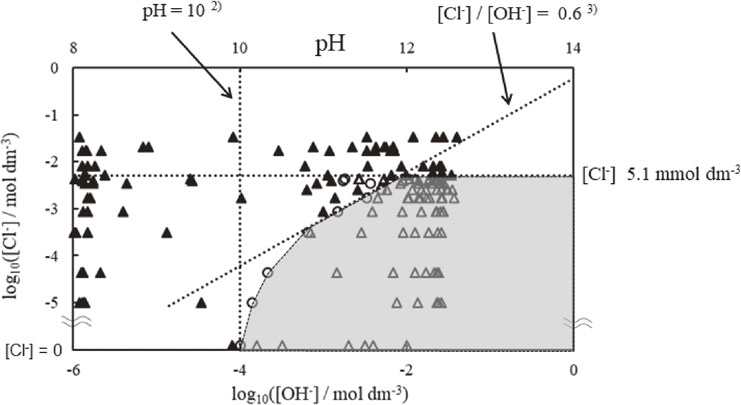
Plots of log10[Cl−] vs. log10[OH−]. The △, ○ and ▲ correspond to the passive state of rebar, de-passivation of rebar, and corrosion of rebar, respectively.
From the relation between chloride ion concentrations and pH in Fig. 6, the occurrence of corrosion of steel rebar embedded in concrete can be discussed as follows:
Therefore, the inside of the dotted line, which shows gray in the figure, composed of plots of de-passivation of steel rebar (○) and chloride ion concentrations of 5.1 mmol/dm3, indicates that the steel rebar in concrete is safe.
In this study, the two frequencies impedance measurement of probe electrodes were performed in simulated concrete pore solution. The air was pumped to the electrolyte solution to simulate the neutralization of concrete during measurement. The Rct estimated by two frequencies impedance measurement and pH of electrolyte solution with different concentrations of chloride ions were monitored to clarify conditions of chloride ions concentrations and pH related to “passive state,” “de-passivation,” and “corrosion” of steel rebar. However, the chloride ions inside concrete where steel rebar is embedded are composed of the free chloride ions and fixed chloride ions. The free chloride ions can move freely in the liquid phase within the void.
Moreover, the fixed chloride ions are taken into the hardened concrete and do not move.11) It is reported that the corrosion of steel rebar in concrete is affected by the free chloride ions in the liquid phase within void.11) In these respects, further investigations are required to clarify the conditions for the safe use of reinforced concrete.
The two frequencies impedance measurement of probe electrodes was performed in a saturated calcium hydroxide solution containing various concentrations of chloride ions. The conditions of concentrations of chloride ions and pH for de-passivation of passive film formed on steel rebar in concrete were investigated by monitoring Rct and pH during this measurement. The findings of the present study are as follows: