2021 Volume 62 Issue 5 Pages 661-666
2021 Volume 62 Issue 5 Pages 661-666
Magnesium tin silicide (Mg2Si1−ySny) is a thermoelectric material with a low environmental load and a high power factor of approximately 400°C. High power factor sintering bodies by pressure-less sintering (PLS) have not been produced as Mg contained in the starting material is likely to be oxidized. A certain amount of tin is used as a sintering additive to improve the sintering density and power factor in liquid-phase sintering and the PLS process (LP-PLS). As a result, LP-PLS bodies with a power factor comparable to that of spark plasma sintering (SPS) bodies were successfully fabricated in this work. No significant changes in thermoelectric characteristics were observed even after heat treatment at 250°C for 32 h in air.
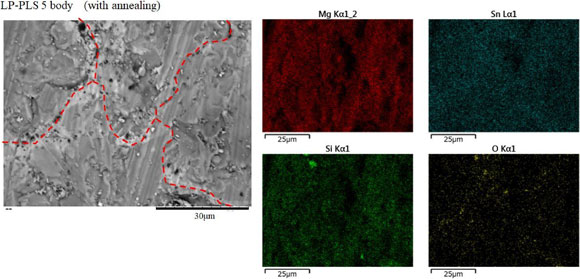
Fig. 6 SEM image and EDX mapping of LP-PLS 5 body.
Materials that possess superior thermal-to-electric energy conversion properties are known as thermoelectric materials. The thermoelectric materials are evaluated by the figure of merit, Z = α2/ρκ, and the power factor (PF) PF = α2/ρ, where α is the Seebeck coefficient, ρ is the resistivity, and κ is thermal conductivity. The thermoelectric material Bi2Te3 has been practically used because of its distinct thermoelectric characteristics.1–3) It is, however, toxic and its natural resources are limited; therefore, it was not suitable for thermoelectric power generation systems. Several kinds of silicides have been investigated for many years because of their low environmental load.
Mg2(Si,Sn)-based compounds are promising materials because of particularly high thermoelectric conversion efficiencies in the temperature range from 300 to 400°C.4–21) Previous reports stated that its oxidation is caused by uncombined Mg.19) It is also known that the thermoelectric characteristics are affected by the Si–Sn ratio.15,16) Special manufacturing methods are required because they are difficult to be sintered.22) For example, spark plasma sintering (SPS),4–10) hot press (HP),11–13) hot isostatic pressing,21) and mechanical alloying14) are utilized in general. SPS provides a high density with a short sintering time, but it restricts the size and shape of the sintering zig. Therefore, it is practically used only for medium or small production. In addition, the production cost increases with the scale of the pressurizer system.
In contrast, the pressure-less sintering (PLS) method is capable of producing a large amount of sintered bodies with high productivity from one mold. PLS has been used for a long time to manufacture a sintered magnet with a density of approximately 90–98% because it provides a desirable shape of the sintered body as the final product. This superiority reduces shaping and matching costs and also the amount of raw materials used. Nishida et al. applied PLS to a thermoelectric material, semiconducting FeSi2,23) and obtained a better thermoelectric ability compared with that of the sintered method.
We also fabricated Mg2(Si,Sn)-based materials using PLS.24) However, the PF was one order smaller than that of the sintered body produced by the SPS method because of the high resistivity resulting from its low sintered density. Furthermore, a single phase of Mg2(Si,Sn) was not obtained because of the high sintering temperature. Consequently, we focused on the liquid-phase sintering method, which can reduce the sintering temperature and is used for sintering ceramics with a high melting point.25–27) Figure 1 shows a schematic illustration of pressure-less sintering (PLS) and liquid-phase pressure-less sintering (LP-PLS) mechanism. The sintering additive increases the diffusion force in the liquid phase at approximately the sintering temperature. The liquid-phase sintering additive in the interparticle at approximately the sintering temperature increased the diffusion force of the sintering particles.
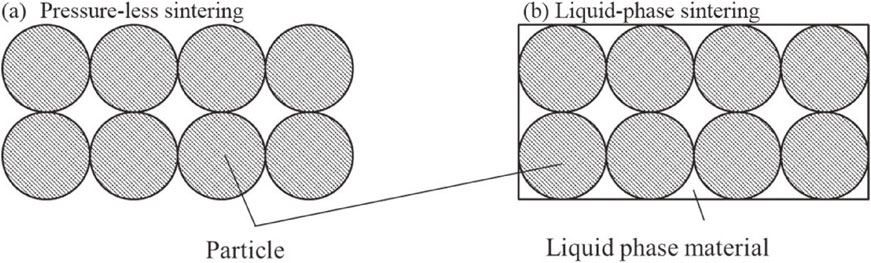
(a), (b) Schematic illustration of pressure-less sintering (PLS) and liquid-phase sintering (LP-PLS) mechanism.
In this study, we examined the production method of Mg2(Si,Sn) sintered bodies by combining the PLS method and the liquid-phase sintering mechanism and also evaluated the thermal stability and thermoelectric properties of the sintered bodies produced by our method. These products will become an independent power source suitable for the Internet of Things (IoT) sensors.
The starting materials consisting of Mg (180 µm, 99.5%), Si (180 µm, 99.9%), Sn (150 µm, 99.9%), and Sb (180 µm, 99.99%) were mixed uniformly and cold pressed at a pressure of 20 MPa and then synthesized into Mg2Sn0.5+xSi0.5−ySby (x = 0 and 3 at%, and y = 0.5 at%) by annealing at 700°C for 4 h in a graphite autoclave under an Ar atmosphere. This process will be termed reaction synthesis (RS). After RS, the synthesized material was ground through a sieve into a powder with a particle size of 38 µm or less. Next, the ground powder mixed with 1 mass% poly vinyl-butyral (PVB) was granulated into particles ranging from 180 to 350 µm in size. The granulated particles were charged in a 33 mm × 4 mm × 4 mm rectangular parallelepiped mold and pressed one-directionally at a pressure of 200 MPa to form Mg2Sn0.5+xSi0.5−ySby molded body. Liquid-phase pressure-less sintering was carried out by sintering the Mg2Sn0.5+xSi0.5−ySby molded body at 850°C for 5 h in an Ar atmosphere. After LP-PLS, the sintered body was annealed at 400°C for 0, 3, and 5 h in an Ar atmosphere.
In the reference SPS body, the grounded powder (38 µm pass) was sintered at 730°C for 15 min under a zig pressure of 50 MPa in a vacuum of 4.6 × 10−4 Pa by SPS (Sinter land LABOX-1575) system. All experimental conditions of the samples in this study are listed in Table 1.
The Seebeck coefficient and electrical resistivity were measured using a ZEM-3 (ADVANCE RIKO ZEM-3). X-ray diffraction (XRD; RIGAKU RINT-2500) was performed to identify the crystalline phases of the starting material powder and the sintered bodies. The material components were identified by Raman spectroscopy (Nano photon RAMAN touch) with a laser wavelength of 532 nm and a diffraction grating at 600 gr/mm. For the thermal stability test, the sintered bodies were maintained at 250°C in air for up to 32 h. The Seebeck coefficients and resistivities of the thermal test bodies were measured at 25°C by removing the test bodies after 0, 2, 4, 8, 16, and 32 h. The sintered bodies were observed by scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX; Hitachi high technologies TM-3030/ Oxford instruments X-stream2).
Table 2 shows the thermoelectric properties of the PLS, LP-PLS, and SPS sintered bodies at 300 K and their appearances after they were maintained in ambient air for 20 days. The resistivity of the PLS Vac. body (Ref. 24)) is one order of magnitude higher than those of other sintered bodies (LP-PLS 1–6 and SPS), and its volume density was significantly lower, although its Seebeck coefficient was the same as that of the SPS body. In contrast, the resistivities and Seebeck coefficients of the sintered bodies synthesized by LP-PLS, where the sintering was carried out in an Ar atmosphere, were approximately equal to those of the SPS body, and their volume densities were as high as that of the SPS body. A difference in PLS Vac. and LP-PLS bodies might be because liquid-phase Sn could not remain inside the PLS Vac body during the PLS in vacuum.

Figures 2(a)–(c) show the temperature dependence of the Seebeck coefficient, resistivity, and PF of PLS, LP-PLS with annealing, and SPS bodies, respectively. The Seebeck coefficient of the SPS body was increased to a value of 20% higher than those of LP-PLS 2 and 5 bodies, while the PF at 400°C was comparable in SPS and LP-PLS 5 bodies. The resistivity of LP-PLS 5 body was lower than that of LP-PLS 2 body because of the excess Sn content, and the PF of LP-PLS 5 body was also increased by 10%.
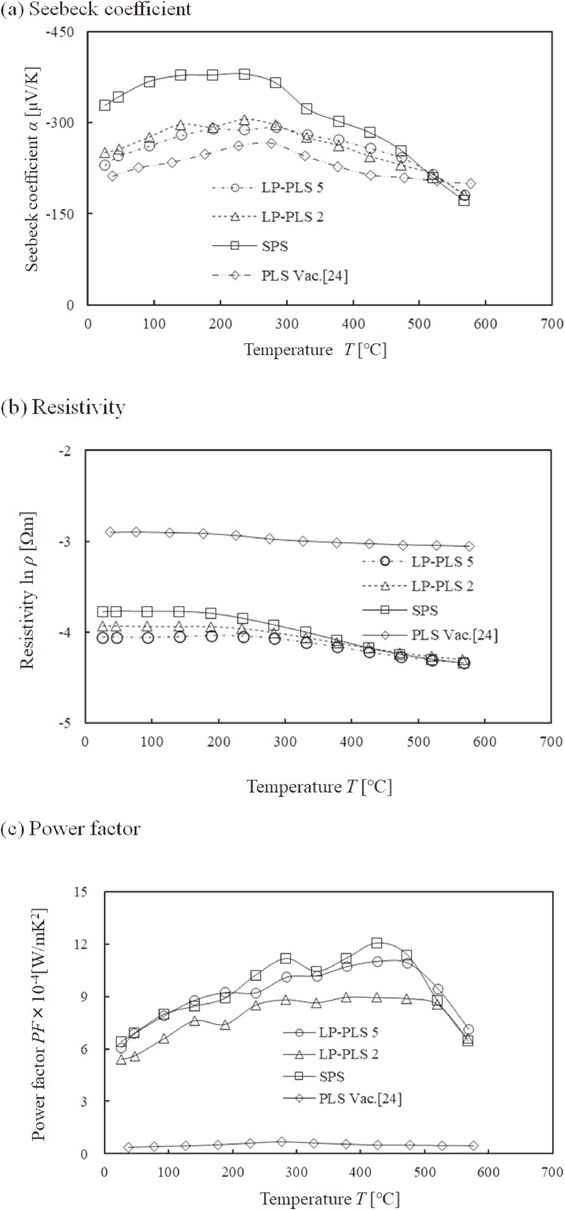
(a)–(c) Thermoelectric properties of Mg2(Si,Sn) sintering bodies.
The excess Sn dispersed in the LP-PLS body as a pure Sn phase, as presented later, reduced resistivity because the resistivity of pure Sn is lower than that of the Mg2(Si,Sn) phase.
The temperature dependence of the overall thermoelectric properties was compatible with the LP-PLS and SPS bodies. The PF values in the LP-PLS 2 and 5 bodies were improved by 35 times compared to that of the conventional PL Vac. sintered body.24)
The heat resistance properties of Mg2(Si,Sn) sintered bodies with SPS or HP were not applied the LP-PLS body because the sintering additive may affect the thermal durability, and further thermal resistance tests are required. Figure 3 shows the time dependence of the Seebeck coefficient and resistivity during their measurements on the LP-PLS 5 body. No significant changes in the time dependence of the Seebeck coefficient and resistivity were observed in the LP-PLS bodies.

Thermoelectric properties of thermal stability test up to 32 hours.
The sintered bodies synthesized by LP-PLS exhibited better thermoelectric properties and thermal durability compared with the SPS body.
3.2 Durability of Mg2(Si,Sn) LP-PLS bodies prepared by LP-PLSFigures 4(a) and (b) show the photographs of LP-PLS bodies annealed (LP-PLS 5) and without annealing (LP-PLS 4) after being maintained under ambient conditions for 20 days. No significant change was found in the appearance in the annealed LP-PLS 5 body, whereas the corner of the side edge was easily broken into a powder in the LP-PLS 4 body without annealing when it was pinched with a tweezer.

(a), (b) Photograph of LP-PLS bodies after 20 days.
Figures 5(a) and (b) show the SEM images of the light-colored and dark-colored parts of the LP-PLS body with annealing (LP-PLS 5), respectively. In the light-colored part, most of the body area consisted of the Mg2Si0.6Sn0.4 phase with a limited amount of MgO phase. In contrast, in the dark-colored part, the area of the MgO phase was larger than that in the light-colored part, and several spherical pure Sn precipitates, which might have been formed due to the melting of excess Sn, were found by the EDX spectrum analysis.

(a), (b) SEM images of LP-PLS bodies with annealing.
Figure 6 shows the EDX-mapping image around the grain boundaries of the Mg2Si0.6Sn0.4 phase and MgO phase in LP-PLS 5. As the mapping illustrates, Sn components were uniformly distributed in the grains, while O components were identified near the grain boundaries. Mg and Si components tended to have high concentrations in the grain.

SEM image and EDX mapping of LP-PLS 5 body.
Figures 7(a)–(b) and Table 3 show the results of the quantitative EDX analysis of the grains and boundaries of the sintered bodies with annealing (LP-PLS 5) and without annealing (LP-PLS 4). The measured Mg, Sn, Si, and Sb compositions inside the grain in LP-PLS 5 were almost the same as those of the preparation composition, while in the boundary, excess Sn and O were identified, suggesting enhanced oxidation at the grain boundary. In the sintered body without annealing, a larger amount of oxygen was found both inside the grain and grain boundary than the bodies with annealing, although the trends of composition ratio were the same.
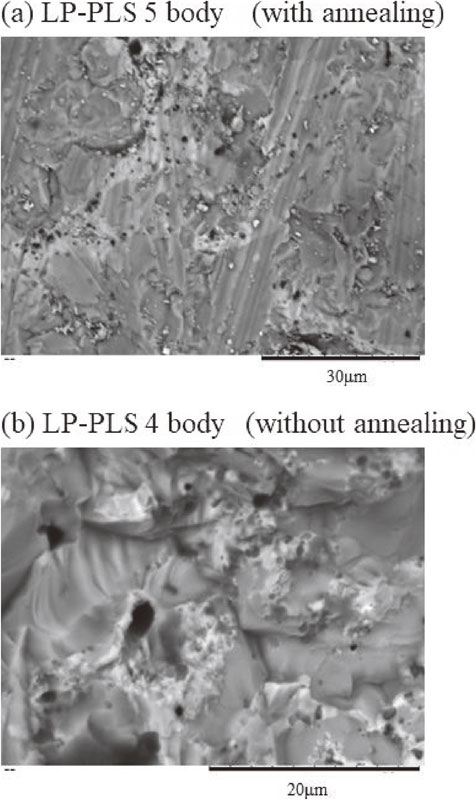
(a), (b) SEM images of LP-PLS bodies.
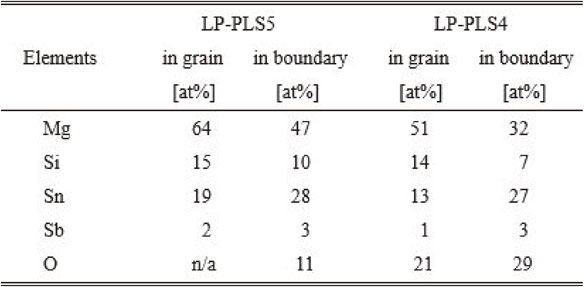
Figure 8 shows the XRD profiles of RS powder and PLS, LP-PLS 2, LP-PLS 5, SPS bodies, and the peak references from the JCPDS data. It was confirmed that the RS powder and SPS body were almost in a single phase of Mg2Si0.4Sn0.6, while the LP-PLS bodies were identified to be in the Mg2Si0.4Sn0.6 and Mg2Sn phases. It is known that phase separation occurs in Mg2(Si,Sn) at elevated temperatures over 700°C.28)
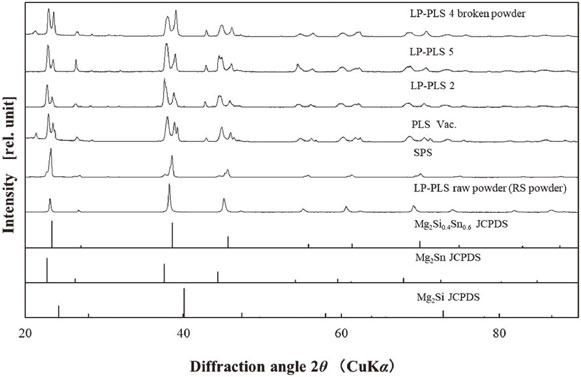
XRD profiles of Mg2(Si,Sn) sintering bodies.
Figure 9 shows the Raman spectroscopy profile of the sintered bodies with annealing (LP-PLS 5) and without annealing (LP-PLS 4). From the Raman profile, Mg2Si, MgO, and SnO2 peaks were detected in both sintered bodies with or without annealing. In addition, for LP-PLS 4, the ratios of the peak intensity of Mg2Si to those of MgO and SnO2 are relatively weak compared with those for LP-PLS 5. This result reveals that the oxidation was significantly enhanced in the sintered bodies without annealing (LP-PLS 4).

Raman spectrum profiles of Mg2(Si,Sn) LP-PLS body and powders.
The experimental results in Fig. 5 to Fig. 9 indicated that the annealing treatment of the LP-PLS body may reduce the pure Sn content, which would infiltrate into the sintered body during LP-PLS inside the grain and form a uniform Mg2(Si,Sn) phase. As shown in Figs. 6 and 7, the pure Sn phase in the sintered body diffused from the inside to the outside of the sintering body via grain boundaries. The pure Sn phase remaining inside the sintered body may oxidize gradually in the atmosphere, which would deteriorate the strength of the sintered body because the Sn oxide components cause internal stress owing to its volume expansion.
In terms of the results, the reduction in the absolute amount of Sn oxide in the sintered body was caused by annealing, which led to the low resistivity and long-term stability of the sintered body with annealing.
We succeeded in fabricating Mg2(Si,Sn) sintered bodies with good PF values, whose values are the same as that of the SPS or HP synthesized Mg2(Si,Sn) bodies, by developing the LP-PLS method. The Sn sintering additive that penetrated the grain boundaries during sintering caused an increase in the relative sintering density and electrical conductivity of the LP-PLS body. The thermoelectric characteristics were stable even after 32 h under the atmosphere at 250°C. The sintering additive of Sn is easy to use because it is a component of the Mg2(Si,Sn) constituent metal.
Our LP-PLS body can be mass-produced at a low cost; thus, the proposed LP-PLS method can be applied to practical production.
We are grateful to Dr. Ohsugi for helpful discussions in this study.