2021 Volume 62 Issue 7 Pages 1030-1038
2021 Volume 62 Issue 7 Pages 1030-1038
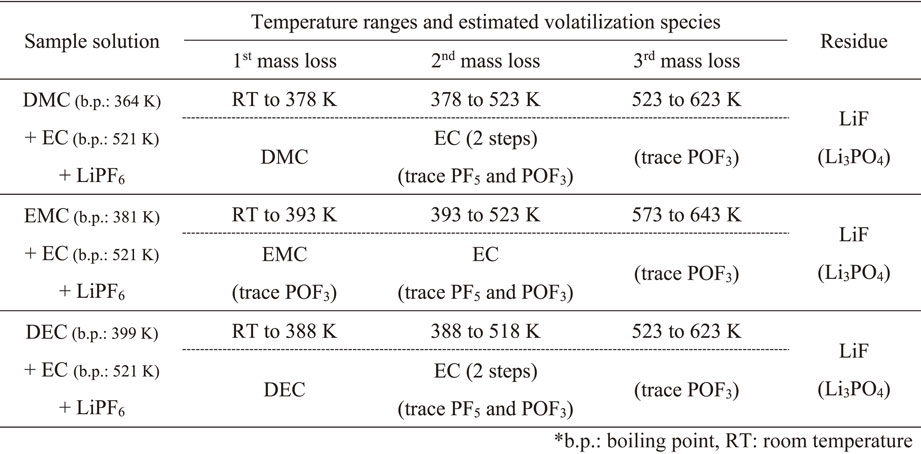
For the distillation separation of organic solvents from lithium-ion battery (LIB) cells and their recycling, the volatilization behavior characteristics of lithium hexafluorophosphate (LiPF6) with several types of alkyl carbonates in open cells were studied using mass spectrometry from 296 to 773 K. Similar volatilization behaviors were observed for three different dialkyl carbonates, i.e., dimethyl carbonate (DMC), ethylmethyl carbonate (EMC), and diethyl carbonate (DEC). When the electrolytic solution from single dialkyl carbonate with LiPF6 was heated, solution mass loss occurred in three steps. In the first step near the boiling points of the dialkyl carbonates, it is expected that dialkyl carbonates (with trace POF3 for DMC and EMC) can be recovered. The residues after heating to 773 K contained LiF and Li3PO4. For electrolytic solution from mixed alkyl carbonates with LiPF6, dialkyl carbonates with higher volatilities could be recovered near their boiling points (with trace POF3 for EMC + ethylene carbonate (EC)). EC with lower volatilities than the dialkyl carbonates could be recovered at higher temperatures near the EC boiling point with trace PF5 and POF3. The residues after heating to 773 K contained LiF and Li3PO4. With moisture, POF3 generation, which must accompany hydrofluoric-acid generation, was observed from a lower temperature than that without moisture. Moisture also affected the chemical form of the residues. Lithium salts, such as Li4P2O7, LiPO3, LiF, or Li3PO4 were observed. The presence of water should be avoided when recovered electrolytic solution from LIBs is separated and recycled by distillation. Oxygen in air does not affect the volatilization behavior of LIB electrolytic solutions. These insights will be of practical importance to consider electrolytic solution recovery from LIB cells and its recycling by distillation without incineration to avoid damage by hydrofluoric-acid generation.
Lithium-ion batteries (LIBs) have many attractive characteristics and are used in various applications, including hybrid electric vehicles, plug-in hybrid electric vehicles, and electric vehicles. The percentage of these next-generation cars as a proportion of newly sold cars has increased. Because of this increasing trend, it is expected that the number of end-of-life LIBs that are generated from waste vehicles will increase in future.
LIBs contain various metal elements, such as Co, Ni, and Li, and thus, a variety of processes have been investigated for their recycling, as summarized in recent reviews.1–3) However, complex treatment costs for LIBs with multiple steps, such as incineration and solvent extraction, are often higher than the values of the contained valuable resources. Thus, end-of-life LIBs are often incinerated, and the resultant slags are used as road-bed materials without any valuable resource recovery. Simpler and more effective recycling processes for LIBs are required.
Honda Motor Co., Ltd.; Japan Metals & Chemicals Co., Ltd.; and Tohoku University investigated an incineration-less recycling system for LIBs with an effective disassembly of LIB units.4) In this recycling system, electrolytic solution, i.e., lithium salt (typically lithium hexafluorophosphate, LiPF6) with organic solvent (alkyl carbonates) inside the LIB cell was drained from the cell in a sealed condition before opening the cell for disassembly. In the drainage step, cleaning solution was injected to rinse the inside of the cell. To minimize environmental impact by additional solvent introduction, a typical electrolyte solvent, such as dimethyl carbonate (DMC), was used as the cleaning solution. Nickel–cobalt alloy was produced from extracted cathodes after disassembly, and it was confirmed that the produced alloy could be used as a metal hydride. Avoidance of electrolytic solution incineration limits corrosive gas production, such as hydrogen fluoride (HF), and avoids severe damage to components inside the LIB cells and to the treatment equipment.
Recovered electrolytic solution with cleaning solvent, which is typically a mixture of LiPF6 and several organic carbonate types, could be separated by distillation for reuse.
A similar concept to recover electrolytic solution by depressurized vaporization with heating has been proposed in the literature;5,6) however, fluorine in LiPF6 is recovered as HF with water addition. To consider the recovery of these electrolyte solvents without HF generation, the vaporization behavior characteristics of this mixture and the influence of humidity must be investigated.
Similar research on the thermal behavior of electrolytic solution, i.e., a mixture of lithium salt and solvents in LIBs cell, has been conducted to study the performance change of batteries at elevated temperatures or its behavior during serious safety problems that are caused by flammable organic solvents.7) Ravdel et al.8) investigated the thermal decomposition of LiPF6 at 358 K as solutions in alkyl carbonates, i.e., ethylene carbonate (EC), DMC, diethyl carbonate (DEC), and ethylmethyl carbonate (EMC), and mixed carbonates. The LiPF6 decomposed to lithium fluoride (LiF) and phosphorus pentafluoride (PF5), and the generated PF5 reacted with the alkyl carbonates to form a variety of decomposition products, including carbon dioxide (CO2), ethers (R2O), alkyl fluorides (RF), phosphorus oxyfluoride (POF3), and fluorophosphates (POF2OR, POF(OR)2). Kawamura et al.9) investigated the thermal stability of alkyl-carbonate mixed solvent with LiPF6 with/without water and found exothermic peaks between 503 and 553 K, which suggests that LiPF6 is related to an exothermic decomposition reaction. They proposed that the decomposition of DEC solvent with LiPF6 occurs as follows:
| \begin{equation} \text{LiPF$_{6}$ (s)} \leftrightarrow \text{LiF (s)} + \text{PF$_{5}$ (g)} \end{equation} | (1) |
| \begin{align} &\text{C$_{2}$H$_{5}$OCOOC$_{2}$H$_{5}$} + \text{PF$_{5}$} \\ &\quad \rightarrow \text{C$_{2}$H$_{5}$OCOOPF$_{4}$} + \text{C$_{2}$H$_{4}$} + \text{HF} \end{align} | (2) |
| \begin{equation} \text{C$_{2}$H$_{5}$OCOOC$_{2}$H$_{5}$} + \text{PF$_{5}$} \rightarrow \text{C$_{2}$H$_{5}$OCOOPF$_{4}$} + \text{C$_{2}$H$_{5}$F} \end{equation} | (3) |
| \begin{equation} \text{C$_{2}$H$_{5}$OCOOPF$_{4}$} \rightarrow \text{POF$_{3}$} + \text{CO$_{2}$} + \text{C$_{2}$H$_{4}$} + \text{HF} \end{equation} | (4) |
| \begin{equation} \text{C$_{2}$H$_{5}$OCOOPF$_{4}$} \rightarrow \text{POF$_{3}$} + \text{CO$_{2}$} + \text{C$_{2}$H$_{5}$F} \end{equation} | (5) |
| \begin{equation} \text{C$_{2}$H$_{5}$OCOOPF$_{4}$} + \text{HF} \rightarrow \text{PF$_{4}$OH} + \text{CO$_{2}$} + \text{C$_{2}$H$_{5}$F} \end{equation} | (6) |
| \begin{equation} \text{C$_{2}$H$_{5}$OH} + \text{C$_{2}$H$_{4}$} \rightarrow \text{C$_{2}$H$_{5}$OC$_{2}$H$_{5}$} \end{equation} | (7) |
Although a variety of investigations have been conducted, these experiments have been conducted in a closed system, e.g., in sealed pans, because they focused on the performance change of batteries at elevated temperatures or its behavior during serious safety problems. Thus, in this study, for the distillation separation of organic solvents recovered from LIB cells and their recycling, a basic study of the volatilization behavior characteristics of LiPF6 with several alkyl carbonate types was conducted in open cells using mass spectrometry from 296 to 773 K. Water and oxygen, which may affect the volatilization behavior and are important for recycling, were also investigated.
Table 1 summarizes the typical electrolytic solution components of the LIBs that were used in the study, i.e., LiPF6, DMC, EMC, DEC, and EC. Battery grade reagents for these alkyl carbonates were from the Wako Pure Chemical Industries Ltd. (Osaka, Japan). Water contents in the DMC, EMC, DEC, and EC were reported by the company to be 12, 16, 10, and 27 ppm, respectively. Battery grade electrolytic solutions of 1.0 mol/L of LiPF6 in DMC/EMC/DEC/DMC + EC: 50/50 (v/v)/EMC + EC: 50/50 (v/v)/DEC + EC: 50/50 (v/v) were from Sigma-Aldrich Co. LLC. (St. Louis, MO, US). Water contents in the electrolytic solution measured by the Karl–Fischer method were reported by the company to be 10, 10, 9, 9, 11, and 5, respectively.
A skimmer-MS system (STA409CD/3/403/5/G, Netzsch GmbH, Selb, Germany, MS: QMG422, Inficon, Bad Ragaz, Switzerland) was used. The skimmer-MS system allows for simultaneous thermal analysis and mass spectrometry with ideal vapor gas transfer into the mass spectrometer under homogenous heating conditions,18,19) and has been used extensively for vapor gas analyses.20–23) Electrolytic solution samples were heated in an Al open cell (φ 6.8 mm, 85 µL) under near-atmospheric-pressure conditions (non-vacuum conditions), and the evaporating gas was analyzed with the mass spectrometer. Changes in electrolytic solution mass were measured during heating. DMC, EMC, and DEC are liquid at room temperature, and 70–85 µL of the electrolyte solvent was collected in a glove box in an Ar atmosphere, and introduced into the Al cell. EC is solid at room temperature. The EC was heated and melted in the Ar atmosphere, and 70–85 µL was collected and introduced into the Al cell. For single- or mixed-electrolyte solvents with LiPF6, 70–85 µL of the electrolytic solution was collected in a glove box in an Ar atmosphere, and introduced into the Al cell. Gas replacement was conducted for 15 min under an Ar flow of 70 mL/min after Al cell setting in the skimmer-MS system. The heating temperature range for the electrolytic solution samples was 296 to 773 K, at 10 K/min. The Ar gas flow rate was set at 70 mL/min. The gas flow was regulated at room temperature, and then introduced to the system in all the experiments. For mass spectrometry, the m/z range was 1 to 200. The ionization voltage was set to 70 eV. The voltage of the secondary electron multiplier was 2000 V. The pressure in the mass spectrometer system was ∼8 × 10−4 Pa. Solid residues in Al cells after heating were analyzed by X-ray diffractometry (XRD, D2 PHASER, Bruker AXS GmbH, Karlsruhe, Germany Radiation source: Cu-Kα).
To investigate the influence of H2O, the volatilization behavior of alkyl carbonates with and without moisture content was examined. In the series of experiments, moisture was introduced by Ar gas bubbling into water. To decrease the background noise from H2O addition, the Ar flow rate was set to 250 mL/min. The estimated introduced H2O was ∼12 g-H2O/m3-Ar from temperature and humidity data that were obtained by a thermohygrometer and the state and Antoine equations. To investigate the O2 influence, air was used instead of Ar, and the gas flow rate was set to 70 mL/min.
When each solvent, i.e., DMC, EMC, DEC, and EC, was heated in an open cell from room temperature to ∼573 K, a uniform mass loss was observed for all solvents near each boiling point until volatilization was completed. Figure 1 shows one of the typical results, namely, the volatilization behavior of DMC. The DMC mass in an open cell decreased uniformly around its boiling point (363.7 K). The solvent volatility and mass losses were not negligible even at room temperature, and thus, it was impossible to determine the initial sample mass accurately in an open cell. Therefore, the vertical axis in Fig. 1 shows mass changes from the beginning of the measurements. Figure 2 shows a typical result, which is the mass spectrum of the DMC vapor at ∼333 K. The spectrum is like that for DMC, which is reported in the mass spectra database.24) No obvious change in mass spectra for the DMC vapor was observed for all studied temperatures. The results were similar for all other solvents, i.e., EMC, DEC, and EC. Therefore, no significant thermal decomposition of the single solvent during its evaporation occurred for the studied temperature range.

Volatilization behavior of DMC (boiling point: 363.65 K).

Mass spectra of DMC vapor at ∼333 K.
When mixed solvents, i.e., EC + DMC, EC + EMC, and EC + DEC, were heated in an open cell from room temperature to ∼573 K, a simple overlap of mass losses and mass spectra by volatilization of each mixed solvent was observed (data not shown). Therefore, it is considered that any interactions between each solvent, such as the reaction between the solvents and their decomposition, did not occur for the studied temperature range.
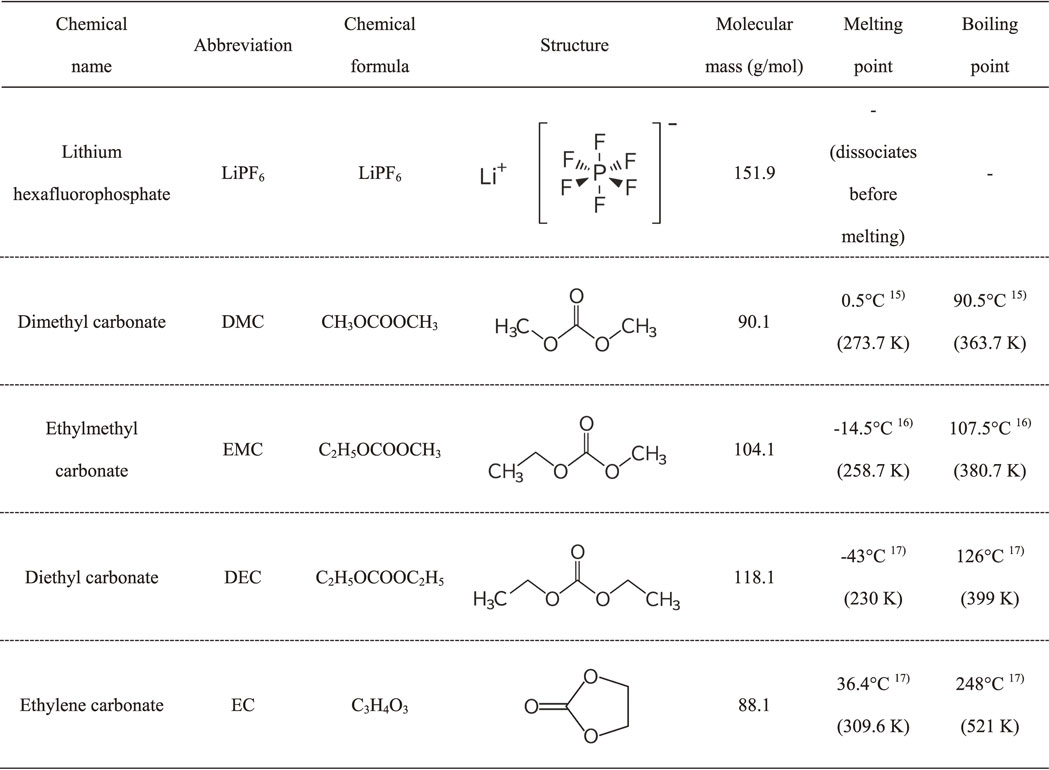
3.2 Volatilization behavior of single alkyl carbonate with LiPF6Similar volatilization behaviors have been observed for all single dialkyl carbonates, i.e., DMC, EMC, and DEC with LiPF6. All mixtures lost mass in three steps and the volatilization mechanism was the same. Table 2 summarizes the volatilization behaviors for these three carbonates with LiPF6. The results for DMC with LiPF6 are described in detail as representative of all dialkyl carbonates. Figures 3 and 4 show the volatilization behavior of DMC with LiPF6 and the ion current for a dominant ionization product of DMC (m/z = 15), PF5 (m/z = 107), and POF3 (m/z = 104) as a function of temperature.


Volatilization behavior of DMC with LiPF6 and ion current for a dominant ionization product of DMC (m/z = 15) as a function of temperature.
Volatilization behavior of DMC with LiPF6 and ion currents for a dominant ionization product of PF5 (m/z = 107) and POF3 (m/z = 104) as functions of temperature.
This mixture lost mass in three steps. First, a large mass loss occurred from room temperature to 391 K. This temperature range corresponds to the boiling point of DMC (363.7 K). The volatilization species in the first step were estimated to be DMC from mass spectra data. Volatilization of trace amounts of POF3 were also observed for DMC and EMC. The ion current for POF3 was lower than those for DMC and EMC. No significant ionization products that can be assigned to other chemicals were observed in the mass spectra data. Ionization products with a m/z larger than 108 were negligible for all samples and the temperature ranges studied. The mass spectra for HF cannot be measured because its mass (m/z = 20) overlapped with that for divalent Ar (m/z = 40/2 = 20), which was used as an atmospheric gas. The generation of POF3 at elevated temperatures was reported in previous research in a closed system.8–11,14) The generation of POF3 is attributed to the following reaction derived from trace water impurity in the electrolytic solution:
| \begin{equation} \text{LiPF$_{6}$} + \text{H$_{2}$O} \rightarrow \text{LiF} + \text{POF$_{3}$} + \text{2HF}. \end{equation} | (8) |
| \begin{equation} \text{PF$_{5}$ (g)} + \text{H$_{2}$O (g)} \rightarrow \text{POF$_{3}$ (g)} + \text{2HF (g)}. \end{equation} | (9) |
A smaller third mass loss occurred from 523 to 623 K. The volatilization species here may be POF3. Further investigation is required; however, the mass loss may be derived from Li salt decomposition.
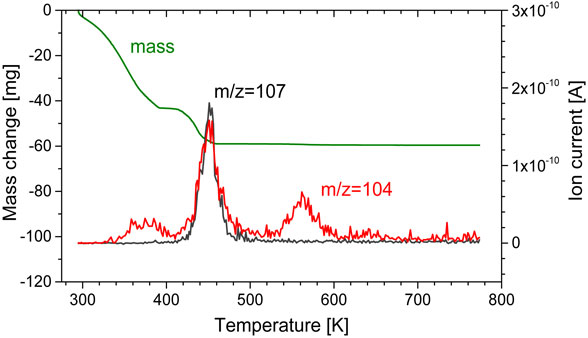
Figure 5 shows the XRD patterns of the residues after heating. For all heating residues, peaks that can be assigned to LiF and Li3PO4 were observed. The peaks that were assigned to Li3PO4 were smaller than those for LiF. LiF generation can be explained from the simple thermal decomposition of LiPF6 as eq. (1). The generation of Li3PO4 has not been reported elsewhere.
XRD patterns of residues after heating for single dialkyl carbonates, symbol ● (LiF, PDF No. 01-070-1934) and △ (Li3PO4, PDF No. 00-025-1030).
If we consider the recycling of recovered electrolytic solution, i.e., a mixture that consists of single dialkyl carbonate (DMC, EMC, or DEC) with LiPF6, more than half of the dialkyl carbonate with trace POF3 can be recovered by volatilization around their boiling points. The rest of the dialkyl carbonate with trace PF5 and POF3 can be recovered in the second volatilization step. However, a heating temperature above the boiling point is required. The third vaporization step produces trace POF3, thus it is considered that the volatilization step must be avoided. The composition of the final solid residue at 773 K was LiF and Li3PO4.
3.3 Volatilization behavior of mixed alkyl carbonates with LiPF6Similar volatilization behaviors were observed for all mixed alkyl carbonates, i.e., DMC + EC, EMC + EC, and DEC + EC with LiPF6. All mixtures lost mass in three steps and the volatilization mechanism was the same. Table 3 summarizes the volatilization behaviors for mixed carbonates with LiPF6. Here, results for DMC + EC with LiPF6 are described in detail as an example. Figures 6 and 7 show the volatilization behavior of DMC + EC with LiPF6 and the ion current for a dominant ionization product of DMC (m/z = 59), EC (m/z = 88), PF5 (m/z = 107), and POF3 (m/z = 104) as a function of temperature. DMC and EC have mass fragments for m/z = 15 and 29. Thus, mass fragments with m/z = 59 and 88 were selected for DMC and EC instead of m/z = 15 and 29, respectively.
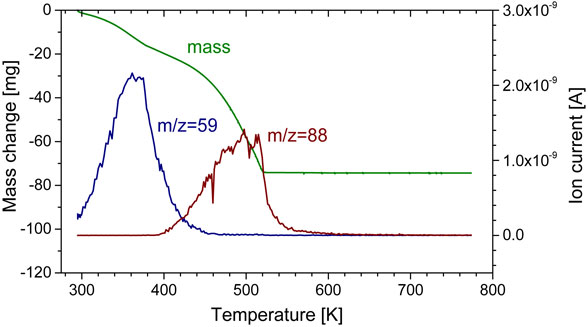
Volatilization behavior of DMC + EC with LiPF6 and ion current for a dominant ionization product of DMC (m/z = 59) and EC (m/z = 88) as a function of temperature.

Volatilization behavior of DMC + EC with LiPF6 and ion currents for a dominant ionization product of PF5 (m/z = 107) and POF3 (m/z = 104) as functions of temperature.
This mixture lost mass in three steps. The first mass loss occurred from room temperature to 378 K. This temperature range corresponds to the DMC boiling point (363.7 K). The estimated volatilization species in the first step was DMC. Volatilization of trace amounts of POF3 was also observed for EMC + EC with LiPF6. No significant ionization products that can be assigned to other chemicals were observed in the mass spectra data. This result indicates that an azeotrope did not form around this temperature.
The second large mass loss occurred from 378 to 523 K. This range corresponds to the EC boiling point (521 K). The volatilization species here can be considered as EC with trace amounts of PF5 and POF3. For single dialkyl carbonates with LiPF6, the dialkyl carbonates volatilized in two steps; however, they volatilized in a single step with EC. The observed peak for the EC-related ion product (m/z = 88) at ∼500 K in Fig. 6 overlapped two peaks with peaks at 503 K and 513 K. A similar result was also observed for DEC + EC with LiPF6. Like the volatilization behavior of single DMC, EMC, or DEC with LiPF6 as described in the former section, EC stabilization with LiPF6 occurred, and the stabilized EC was released with LiPF6 decomposition with an increase in temperature. These results are consistent with previous studies that reported strong solvation of EC with lithium ions.25,26)
The generation of trace PF5 and POF3 may be derived as shown in eqs. (1), (8), and (9).
A smaller third mass loss occurred from 523 to 623 K. The volatilization species here may be POF3. Further investigation is required; however, the mass loss may be derived from the decomposition of residue that was derived originally from LiPF6.
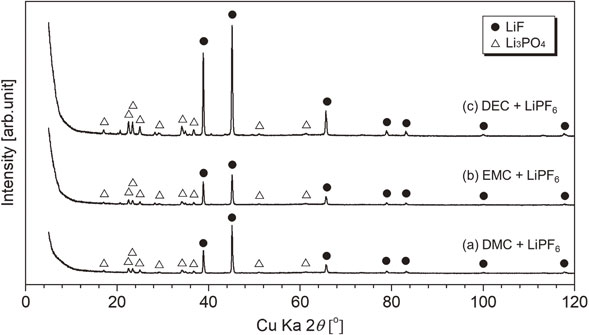
Figure 8 shows XRD patterns of the residues after heating. For all heating residues, peaks that can be assigned to LiF were observed. Small peaks that were assigned to Li3PO4 were observed. LiF generation can be explained from the simple thermal decomposition of LiPF6 as eq. (1).
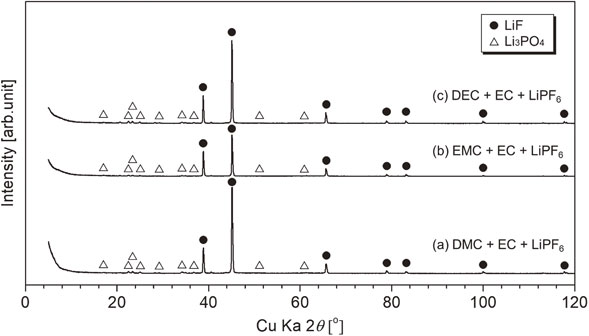
XRD patterns of residues after heating for mixed alkyl carbonates, symbol ● (LiF, PDF No. 01-070-1934) and △ (Li3PO4, PDF No. 00-025-1030).
If we consider the recycling of recovered electrolytic solution, i.e., the mixture of mixed alkyl carbonate (DMC + EC, EMC + EC, or DEC + EC) with LiPF6, dialkyl carbonates with lower boiling points, i.e., DMC, EMC, and DEC, can be recovered by volatilization around their boiling point. The remaining EC evaporated, probably in two steps. In the latter volatilization step, EC was recovered with trace PF5 and POF3. Further heating above 523 K produced trace POF3 vapor. The composition of the final solid residue at 773 K was LiF with trace Li3PO4.
3.4 Influence of H2O on volatilization behavior of alkyl carbonatesThe volatilization behaviors of DMC + LiPF6 with/without moisture were compared at the increased Ar gas flow rate of 250 mL/min. Figure 9 shows the results. Although the observed peak shape and ion current values changed slightly from the former results shown in Figs. 3 and 4, a similar DMC volatilization behavior was observed without moisture. With moisture, a large mass loss occurred between room temperature to 393 K, a slight mass loss occurred at ∼580 K, and a modest mass decrease up to 773 K was observed. The mass loss that corresponds to the second large mass loss that was derived from the second DMC volatilization without moisture was not observed. Therefore, DMC was vaporized in a single step with moisture. Ionic currents from PF5-related products were not observed. The POF3-related mass showed an increase in ionic strength from lower temperatures compared with no H2O addition, i.e., ∼390, 580, and 710 K. It is speculated that the HF is generated with POF3 generation as shown in eqs. (8) and (9). Thus, with moisture, HF can be generated from a lower temperature range. Figure 10 shows the XRD patterns of the residue after heating. The residue contains Li4P2O7, LiPO3, and LiF. Li3PO4, which was observed for the residue without moisture, was not detected. Thus, moisture can affect the residual solid composition.
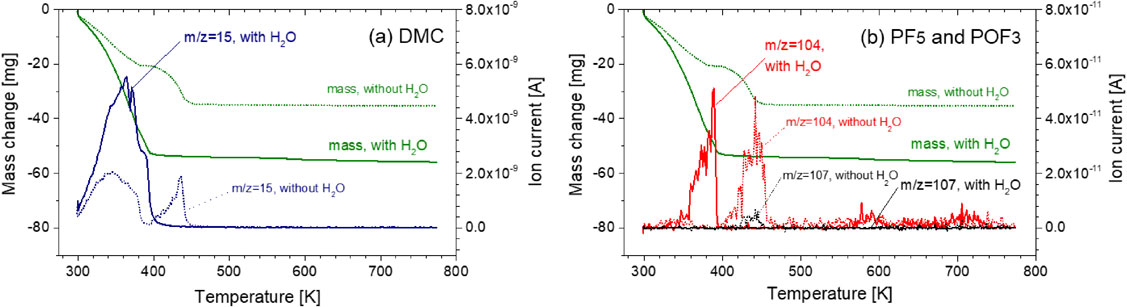
Volatilization behavior of DMC with LiPF6 and ion current for a dominant ionization product of (a) DMC (m/z = 15), (b) PF5 (m/z = 107), and POF3 (m/z = 104) with/without H2O as a function of temperature (solid lines are with H2O and dotted lines are without H2O).
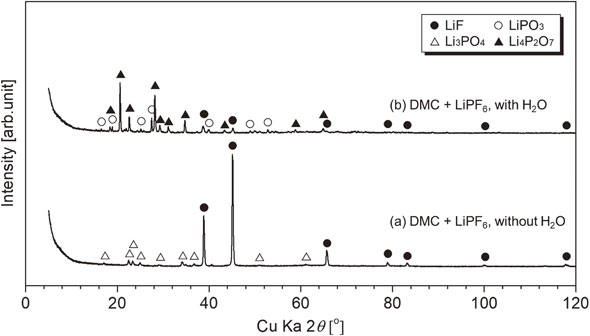
XRD patterns of residues after heating for DMC + LiPF6 with/without H2O; symbol ● (LiF, PDF No. 01-070-1934), △ (Li3PO4, PDF No. 00-025-1030), ○ (LiPO3, PDF No. 01-070-5642), and ▲ (Li4P2O7, PDF No. 01-077-1045).
Figure 11 shows the volatilization behaviors of DMC + EC + LiPF6 with moisture. Data are not shown, but the similar volatilization behaviors of DMC and EC were observed without moisture as shown in Figs. 6 and 7 (the Ar flow rate of the experiment without moisture was 250 mL/min to decrease the background noise from H2O addition, and was different from that of 70 mL/min for the experiments shown in Figs. 6 and 7). A large mass loss between room temperature and 383 K and further mass loss between 383 and 493 K were observed. In addition to the large mass losses, a slight mass loss between 558 and 598 K and a subsequent modest mass decline up to 773 K were observed. The ion current for a dominant ionization product of DMC (m/z = 59) shows that the DMC vaporized in a single step around its boiling point (364 K) as occurred without moisture. The ion current peak for a dominant ionization product of EC (m/z = 88) appeared at ∼483 K, but the peak separation was unclear compared with the case without moisture. Ionic currents that were derived from PF5-related products were not observed. An increase in the POF3-related ionic current was observed at ∼460, 580, and 730 K. The temperature range for the first peak at ∼460 K was much lower than that without moisture. Therefore, HF generation occurred from a lower temperature range with moisture.

Volatilization behavior of DMC + EC with LiPF6 and ion current for a dominant ionization product of (a) DMC (m/z = 59), (b) EC (m/z = 88), (c) PF5 (m/z = 107), and (d) POF3 (m/z = 104) with/without H2O as a function of temperature (solid lines are with H2O and dotted lines are without H2O).
These results show that moisture does not affect the volatilization behavior of DMC with a lower boiling point, but it affects the EC with a higher boiling point. Moisture enhanced the reaction of eq. (8), and POF3 was generated before LiPF6 pyrolysis as shown in eq. (1). POF3 generation at ∼580 K may result from Li salt decomposition. Figure 12 shows the XRD patterns of the residue after heating. The residue contains Li4P2O7, LiPO3, and LiF. Li3PO4 in the residue without moisture remained undetected. Thus, moisture can affect the residual solid composition.
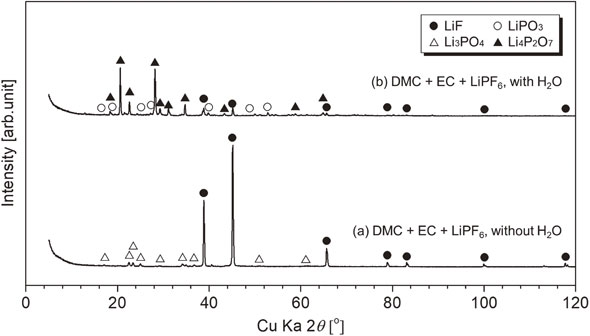
XRD patterns of residues after heating for DMC + EC + LiPF6 with/without H2O; symbol ● (LiF, PDF No. 01-070-1934), △ (Li3PO4, PDF No. 00-025-1030), ○ (LiPO3, PDF No. 01-070-5642), and ▲ (Li4P2O7, PDF No. 01-077-1045).
With moisture, POF3 generation, which must accompany HF generation, was observed from a lower temperature than for that without moisture. High-purity solvent recovery becomes impossible and HF damage becomes possible. Moisture also affects the chemical form of the residual lithium salts. Thus, water should be avoided for recovered electrolytic solution recycle from LIBs by distillation.
3.5 Influence of O2 on volatilization behavior of alkyl carbonatesThe volatilization behaviors of DMC/DMC + EC with LiPF6 with/without oxygen were investigated. Figures 13 and 14 show that no significant differences were observed. Thus, the influence of oxygen in air on the volatilization behavior of alkyl carbonates is small.
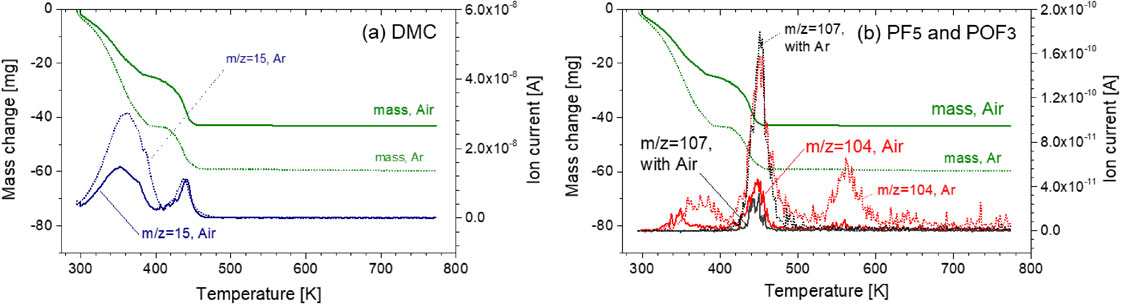
Volatilization behavior of DMC with LiPF6 and ion current for a dominant ionization product of (a) DMC (m/z = 15), (b) PF5 (m/z = 107), and POF3 (m/z = 104) with/without O2 as a function of temperature (solid lines are for Air (with O2) and dotted lines are for Ar (without O2)).
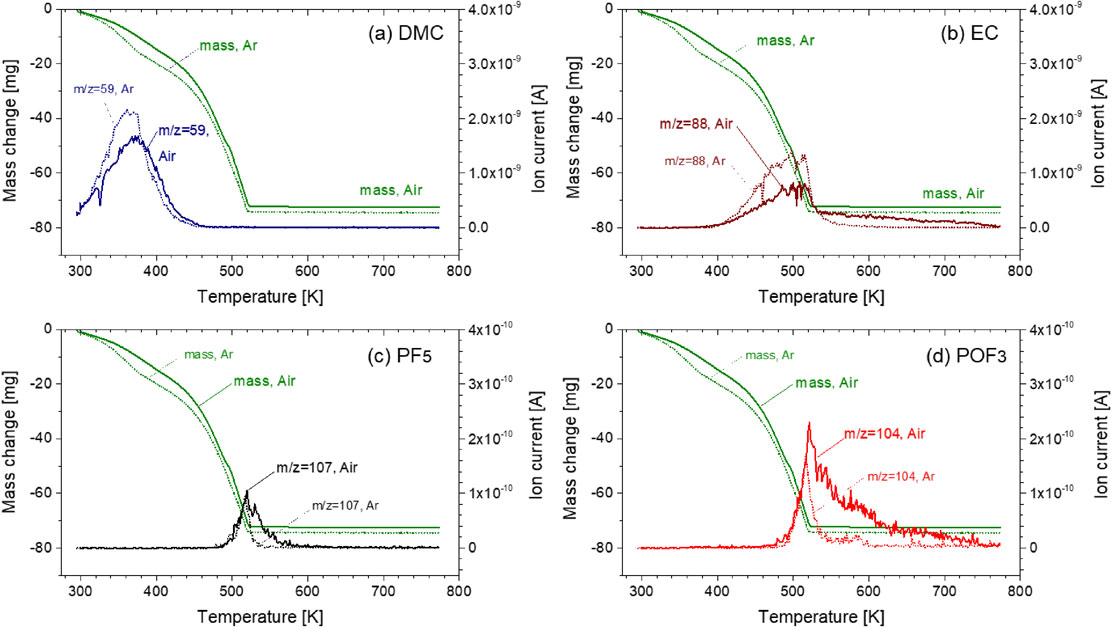
Volatilization behavior of DMC + EC with LiPF6 and ion current for a dominant ionization product of (a) DMC (m/z = 59), (b) EC (m/z = 88), (c) PF5 (m/z = 107), and (d) POF3 (m/z = 104) with/without O2 as a function of temperature (solid lines are with O2 and dotted lines are without O2).
Figure 15 shows the XRD patterns of residues after heating for DMC + LiPF6/DMC + EC + LiPF6 in air (see Figs. 5(a) and 8(a) for residues from the same targets without O2). Even with O2 in air, the chemical form of the residual lithium salts was not influenced.
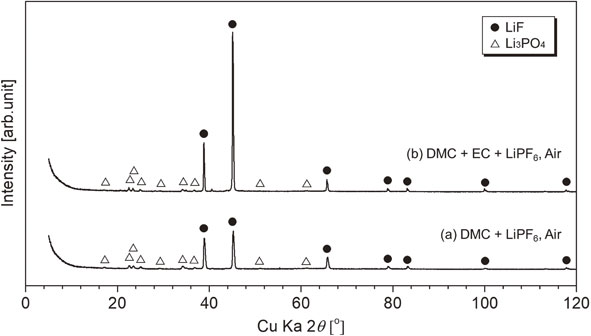
XRD patterns of residues after heating for DMC + LiPF6/DMC + EC + LiPF6 in air, symbol ● (LiF; PDF No. 01-070-1934) and △ (Li3PO4; PDF No. 00-025-1030).
Therefore, oxygen in air does not affect the volatilization behavior of the LIB electrolytic solutions extensively, and operation of the recovery process in air is possible.
For the distillation separation of organic solvents recovered from LIB cells and their recycling, we conducted a basic study of the volatilization behavior characteristics of LiPF6 with several types of alkyl carbonates in open cells with mass spectrometry over a wide temperature range.
Similar volatilization behaviors were observed for three different dialkyl carbonates, i.e., DMC, EMC, and DEC. When electrolytic solution from a single dialkyl carbonate with LiPF6 was heated, the solution lost mass in three steps. In the first step near the boiling points of the dialkyl carbonates, it is expected that dialkyl carbonates (with trace POF3 for DMC and EMC) can be recovered. The residues after heating to 773 K contained LiF and Li3PO4. When the electrolytic solution of mixed alkyl carbonates with LiPF6 was heated, dialkyl carbonates with higher volatilities (with trace POF3 for EMC + EC) could be recovered near their boiling points. EC with lower volatilities than DMC, EMC, and DEC could be recovered at a higher temperature near the EC boiling point with trace PF5 and POF3. The residues after heating to 773 K contained LiF and Li3PO4. In both cases, carbonate stabilization with lower volatilities with lithium salts was observed.
With moisture, POF3 generation, which accompanies HF generation, was observed from a lower temperature compared with that without moisture. Moisture also affects the chemical form of the residues, and lithium salts, such as Li4P2O7, LiPO3, LiF, and Li3PO4 were observed. Water should be avoided in the recycling of recovered electrolytic solution from LIBs by distillation. Oxygen in air does not affect the volatilization behavior of electrolytic solutions of LIBs significantly.
These insights are of practical importance to consider electrolytic solution recovery from LIB cells and recycling by distillation without incineration to avoid damage by HF generation.
This research was supported by the Environment Research and Technology Development Fund (3K152013, principal research institution: Honda Motor Co., Ltd.) of the Ministry of the Environment, Japan. The skimmer mass system used in the study was shared equipment from the High Efficiency Rare Elements Extraction Technology Area in the Tohoku Innovation Materials Technology Initiatives for Reconstruction from the Ministry of Education, Culture, Sports, Science and Technology in Japan.