2022 Volume 63 Issue 2 Pages 118-127
2022 Volume 63 Issue 2 Pages 118-127
High thermal conductivity, high strength and non-flammability have successfully been achieved concurrently for the first time in Mg–4.5Al–2.5Ca, Mg–5Al–3Ca, Mg–6Al–4Ca, and Mg–4Al–2Ca–0.03Be (at%) alloys, which are produced by hot extrusion of the heat-treated cast alloys. These alloys consist of α-Mg, and C36, C14 and C15 compounds, and exhibit a high thermal conductivity of 111–119 Wm−1K−1, a high ignition temperature of 1343–1408 K and a high tensile yield strength of 318–363 MPa.

Comparison of the thermal conductivity and tensile yield strength of the extruded Mg–Al–Ca alloys developed in this study, with various wrought Mg alloys having high thermal conductivity. Only the developed alloys had non-flammability. High thermal conductivity, high strength and non-flammability have successfully been achieved concurrently for the first time in the developed alloys.
Lightweight magnesium (Mg) alloys are very attractive in such applications as automotive, railway, aerospace and 3C (computer, communication and consumer electronics) products.1–3) Especially, with high integration in 3C products, heat radiation and unwavering safety against fire or explosions are more urgently required. Current commercial Mg alloys, e.g., Mg–9Al–1Zn in mass% (AZ91), however, have low thermal conductivity, low yield strength and an unacceptably low ignition temperature.2) There is an increasing demand for Mg alloys with simultaneous achievement of high thermal conductivity, high mechanical strength and non-flammability is increasing.
Magnesium alloys with high thermal conductivity are mainly divided into Mg–Zn, Mg–Al and Mg–Mn alloys.4) All alloying elements tend to have an adverse influence on the thermal conductivity, because the solute atoms could induce the lattice distortion of the α-Mg matrix, change the lattice parameter ratio c/a, and shorten the mean free path of electrons and phonons by substituting Mg atoms.5–8) In the Mg–X binary system, several alloys have a high thermal conductivity of approximately 100 Wm−1K−1; they are Mg–6.5Zn, Mg–1.5Al and Mg–1.5Mn (mass%).4,9,10) In atomic %, these alloys are Mg–2.52Zn, Mg–1.35Al and Mg–0.67Mn. Unfortunately, these high thermal conductivity Mg alloys seem to have a low ignition temperature, because the Zn, Al and Mn elements have no effect on ignition temperature enhancement.11) The improvement of ignition temperature of Mg alloys has been achieved by the addition of Ca, CaO or RE (RE: rare earth) elements, which form a CaO or a REmOn oxide film instead of a MgO film on the surface.12–14) Recently, Mg–1Yb, Mg–1Ca and Mg–1Zn–2Y–xX (X = 0.1Yb, 0.03Be or 1.0Ca) (at%) alloys have been reported to exhibit a high ignition temperature of approximately 1280 K or higher,11,15) which is higher than the maximum oil burner flame temperature (1238 K) in the Mg combustion test standardized by the U.S. Federal Aviation Administration (FAA).16) The individual addition of Yb or Ca, or the simultaneous addition of Yb/Ca, Yb/RE, Be/RE, Be/Ca or Ca/RE is required for the achievement of non-flammability. Addition of alloying elements is a common way to improve the mechanical strength of pure Mg, but the addition generally decreases its thermal conductivity.4) Therefore, it seems to be difficult to simultaneously achieve a high mechanical strength and a high thermal conductivity. For example, a Mg–1Zn–2Y (at%) alloy with a long period stacking ordered (LPSO) phase has a high tensile yield strength of 375 MPa and a high ignition temperature of 1150 K,17–20) but its thermal conductivity is only 52 Wm−1K−1.21) Previously, we have reported that Mg–20Al–10Ca and Mg–10Al–5Ca (at%) alloys produced by rapidly solidified powder metallurgy processing exhibited a tensile yield strength of 500 MPa or higher.22,23) Moreover, we have reported that a wrought Mg–10Al–5Ca alloy, which was produced by extrusion of its cast billet, had a very high ignition temperature around the boiling point of pure Mg (1364 K) and a high tensile yield strength above 400 MPa.24–26) Recently, we have found that the Mg–10Al–5Ca cast alloy heat-treated at 673 K for 2.5 h exhibited a high thermal conductivity of 101 Wm−1K−1 in spite of the large concentration of alloying elements.27) Mg–Al–Ca alloys, therefore, have great potential to simultaneously achieve high thermal conductivity, high mechanical strength and non-flammability.
In this study, we investigated the thermal conductivity, tensile yield strength and ignition temperature of Mg–Al–Ca alloys in order to develop new Mg alloys with high thermal conductivity, high mechanical strength and non-flammability.
Forty-six Mg–Al–Ca alloys were prepared by the high-frequency induction melting of pure Mg (99.99 mass%), Al (99.99 mass%), Ca metals (99.9 mass%) and an Al–2.95 mass% Be master alloy. The molten alloys were maintained at 1023 K for 15 min in an Ar atmosphere and then cast into a mild steel mold. Heat treatment of the as-cast alloys was performed by tube furnace. Alloy ingots (diameter of 30 mm and length of 65 mm) were heated to 673 K for 2.5 h, and then cooled in water or a furnace. The heat-treated alloys were cut into a diameter of 29 mm and a length of 65 mm. The extrusion was conducted at an extrusion ratio of 10 or 20, a temperature of 623 or 673 K, and a ram speed of 2.5 mm s−1. In this study, the terms as-cast, heat treatment with subsequent water cooling, heat treatment with subsequent furnace cooling, and extrusion are represented by AC, HT/WC, HT/FC and Ex, respectively.
The thermal conductivity of the Mg–Al–Ca alloys was measured at 298 K by a thermal conductivity measuring apparatus (Netzsch, LFA 467 HyperFlash). The specimen dimensions were a diameter of 8 mm and a thickness of 2 mm. Prior to the measurement, the surface of the disc specimens was blackened by a carbon coating to increase the absorption of a flash light pulse. A xenon flash lamp was used in order to provide the light pulse. The temperature of the specimen surface was measured by an InSb sensor. The ignition temperature was measured by a thermogravimetry/differential thermal analysis apparatus (SII, TG/DTA-6300). Disc-shaped specimens (diameter of 3.8 mm and thickness of 0.6 mm) with surfaces ground by #4000 SiC paper were placed in an alumina pan and then heated at a rate of 50 K/min in an ambient atmosphere. The specimen temperature was measured using a thermocouple installed underneath the alumina pan. To evaluate the mechanical property of the extruded alloys, a tensile test was performed at a strain rate of 5 × 10−4 s−1. The dimensions of the tensile specimens used in this study were a diameter of 2.5 mm and a gauge length of 12.5 mm, and the tensile direction was parallel to the extrusion direction. The tensile yield strength was obtained from the 0.2% proof stress. The thermal conductivity, tensile properties and ignition temperature were measured for three specimens and averaged. The microstructure was investigated by X-ray diffractometry (Bruker, D8 Discover) and a SEM (JEOL, JIB-4601F) equipped with an energy-dispersive X-ray spectroscopy (EDS) system. Before the XRD measurements and SEM/EDS observations, specimens were cut to a width of 4 mm, a length of 8 mm, and a thickness of 2 mm. XRD patterns were measured at 2θ diffraction angles of 20 to 90 degrees. The step width and counting time for each step were 0.02 degrees and 1 s, respectively. The microstructure was observed by the SEM in the back scatter mode. The solute element concentration in the α-Mg matrix was measured by point analysis of EDS. Microstructural analyses of the alloys were conducted using an aberration-corrected scanning transmission electron microscope operating at 200 kV (STEM/TEM) (JEOL, JEM-ARM200FC). The convergent semi-angle was 20 mrad. The collection semi-angle range of high angle annular dark field (HAADF)-STEM images was 90–175 mrad. The thin foils of the alloys for the structural analysis were prepared using mechanical polishing followed by ion milling (Gatan, PIPS model 1691). The HAADF-STEM images were filtered using the maximum-entropy deconvolution and the realtime up-sampling noise filter to improve the image quality without periodic artifacts (HREM Research, DeConvHAADF and Filter Pro).28) The STEM image simulation was conducted by the multislice method (HREM Research, xHREM).29)
Figure 1 shows the thermal conductivity of the AC and the HT/WC Mg–yAl–xCa (at%) alloys. In the AC state, high thermal conductivity was obtained for lower concentrations of alloying elements. The thermal conductivity of almost all of the Mg–yAl–xCa alloys was improved by heat treatment at 673 K for 2.5 h. Constituent phase obtained from X-ray diffraction patterns30) were divided into three compositional regions of α-Mg, α-Mg+C36, and α-Mg+C36+C14 for the AC alloys, and α-Mg, α-Mg+C36+C15, and α-Mg+C36+C14+C15 for the HT/WQ alloys, respectively. In the HT/WC state, the compositional region having a thermal conductivity of 101 Wm−1K−1 or higher was 2x − 4 < y < 2x + 1 (at%) in the Mg–yAl–xCa alloys, which mainly consisted of α-Mg+C36+C14+C15. Especially, a high thermal conductivity above 111 Wm−1K−1 was obtained along the composition line of Mg–(x + 2)Al–xCa. The study investigated the Mg–(x + 2)Al–xCa (at%) alloys in which x ranged from 0 to 8 at%. Figure 2 shows the thermal conductivity of the AC and the HT/WC Mg–(x + 2)Al–xCa alloys. In the AC state, the thermal conductivity was 73 to 96 Wm−1K−1, and decreased with increasing Ca and Al contents. With regard to the HT/WC treatment, the Mg–(x + 2)Al–xCa alloys with Ca of 2 at% or higher exhibited an improved thermal conductivity. Especially, a high thermal conductivity ranging from 112 to 117 Wm−1K−1 was obtained for the HT/WC alloys with Ca of 2 to 5 at%. The increment in the thermal conductivity brought about by the HT/WC treatment was 23 to 32 Wm−1K−1. Figure 3 shows the ignition temperature of the AC Mg–(x + 2)Al–xCa alloys. In general, the ignition temperature depends on alloy composition without depending on microstructure, because ignition occurs above the liquidus temperature. With increasing Ca content, the ignition temperature increased and then saturated at around the boiling temperature of pure Mg (1364 K).2) It is clear that the AC Mg–(x + 2)Al–xCa alloys with Ca of 2.5 at% or higher exhibited a high ignition temperature above 1238 K, which is the maximum oil burner flame temperature of the FAA Mg combustion test. Especially, the ignition temperature for Ca above 3 at% exceeded the boiling point of pure Mg. Figure 4 shows the tensile mechanical properties of the Mg–(x + 2)Al–xCa alloys produced by hot extrusion of the heat-treated billet (HT/WC+Ex). With increasing Ca content from 0 to 8 at%, the extruded Mg–(x + 2)Al–xCa alloys exhibited a tensile yield strength increase from 160 to 440 MPa and an elongation decrease from 14.7 to 0.6%. Especially, the tensile yield strength and elongation for Ca of 3 to 5 at% was 351 to 380 MPa, and 2.1 to 3.3%, respectively. The thermal conductivity, ignition temperature, and tensile mechanical properties of the Mg–(x + 2)Al–xCa (2 ≤ x ≤ 5 at%) alloys were summarized in Table 1.
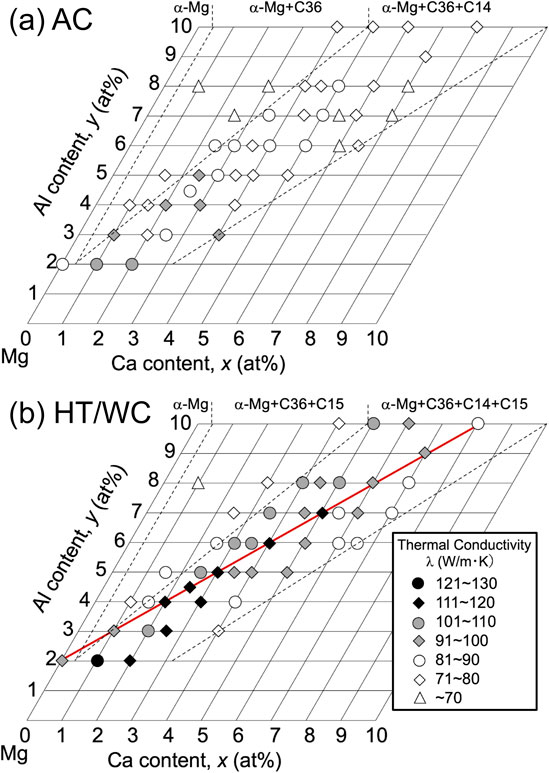
Thermal conductivity of the Mg–yAl–xCa alloys in (a) as-cast (AC) and (b) water-cooling heat-treated (HT/WC) states.
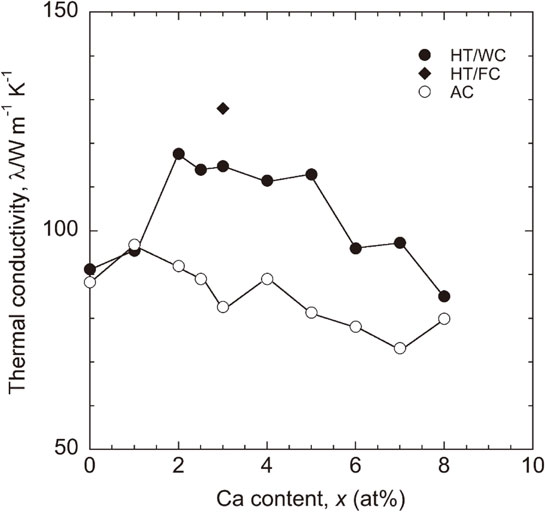
Thermal conductivity of the Mg–(x + 2)Al–xCa alloys in as-cast (AC) and water-cooling heat-treated (HT/WC) states, as well as the thermal conductivity of the Mg–5Al–3Ca alloy in a furnace-cooling heat-treated (HT/FC) state.
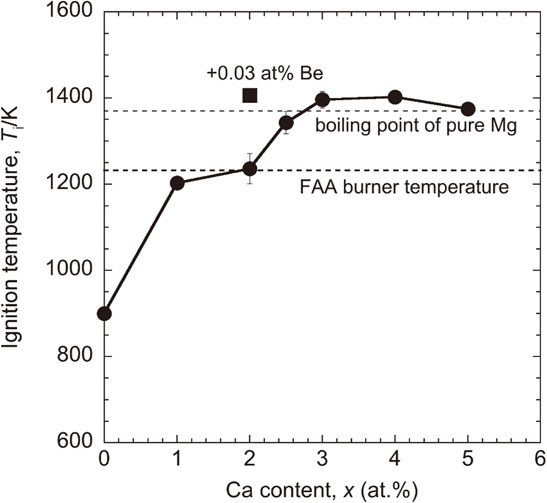
The ignition temperature of the as-cast Mg–(x + 2)Al–xCa and Mg–4Al–2Ca–0.03Be alloys (AC).

Tensile yield strength and elongation of the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex and HT/FC+Ex).

Figure 5 shows the X-ray diffraction patterns of the AC and the HT/WC Mg–(x + 2)Al–xCa alloys. Except for the Ca-free alloy with only α-Mg, the alloys consisted of α-Mg+C36+C14 in the AC state, and α-Mg+C36+C14+C15 in the HT/WC state.30) In the AC state, eutectic lamella structure of α-Mg and C36/C14 compounds increased from 0 to 81% in area fraction with increasing Ca content from 0 to 8 at%, as shown in Fig. 6. Figure 7 shows SEM micrographs of the AC, the HT/WC and the HT/WC+Ex Mg–5Al–3Ca alloys. The eutectic lamellar structure was broken and the lamellar C36/C14 compounds spheroidized by the HT/WC treatment. Many needle- or plate-shaped precipitates, which are considered to be a C15 compound, were observed in the α-Mg matrix of the HT/WC Mg–(x + 2)Al–xCa alloys. The eutectic C36/C14 compounds were broken down into smaller pieces and dispersed in the α-Mg matrix by the hot extrusion. Figures 8(a) and 8(b) show HAADF-STEM images revealing the needle- or plate-shaped precipitates distributed in the α-Mg grain, which are projected along the zone axis $[11\bar{2}0]$α and tilted from the zone axis by 25° on the axis $[1\bar{1}00]$α, respectively. The bright Z-contrast of the precipitates means that the precipitates include heavier elements of Al and Ca. The shape of the precipitates is 4–8 nm in thickness, 0.6–2.0 µm in length, and 1 µm in width. Figure 8(c) is an SAED pattern of the C15 compound precipitated in the α-Mg grain of the alloy. The pattern can be identified with only the index of α-Mg, although there are many precipitations. Figure 8(d) depicts a kinematically simulated SAED of the alloy assuming that the orientation relationship was $(11\bar{1})$C15//(0001)α, $[1\bar{1}0]$C15//$[10\bar{1}0]$α, however the diffraction spots of the C15 compound are not able to be identified by overlapping on the brighter spots of the α-Mg matrix. Figure 9(a) shows the HAADF-STEM image around the interface between the precipitates and the α-Mg. The thickness of the precipitates is 8 nm, which is coincident with the estimation in Fig. 8(a). The interface is atomically sharp and the orientation relationship is $(11\bar{1})$C15//(0001)α, $[1\bar{1}0]$C15//$[10\bar{1}0]$α. The magnified image of the precipitate in Fig. 9(a) is well coincident with the simulated HAADF-STEM image in Fig. 9(c), which was simulated using the multislice simulation technique for the model structure of C15 (Al2Ca) in Fig. 9(d).31) Here, the Z-contrast of Al columns is changing alternately in the $[1\bar{1}0]$ direction because the number of Al atoms is different. These results confirm that the plate-shaped precipitates are the C15 (Al2Ca) compound. Accordingly, it was revealed that plate-shaped fine C15 compounds with a thickness of 4 to 8 nm, a length of 0.6 to 2.0 µm and a width of approximately 1 µm were precipitated on the basal plane of the α-Mg matrix by the heat treatment.

X-ray diffraction patterns of the Mg–(x + 2)Al–xCa alloys in (a) as-cast (AC) and (b) water-cooling heat-treated (HT/WC) states.

Cross-sectional SEM micrographs of the Mg–(x + 2)Al–xCa alloys in as-cast (AC) state.
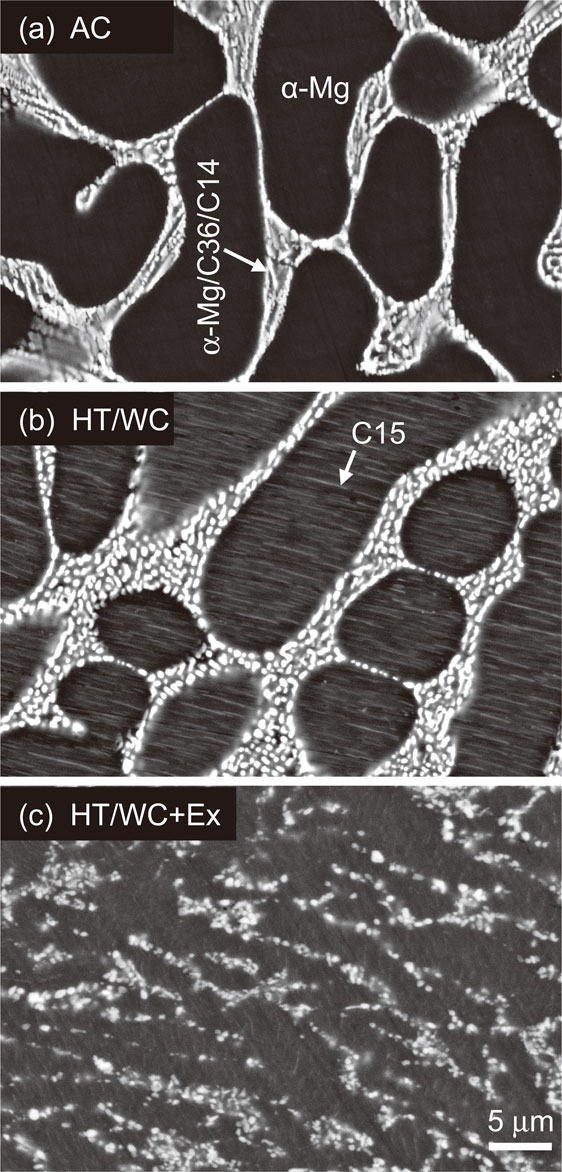
Cross-sectional SEM micrographs of the Mg–5Al–3Ca alloy in (a) as-cast (AC), (b) water-cooling heat-treated (HT/WC) and (c) extruded (TH/WC+Ex) states.
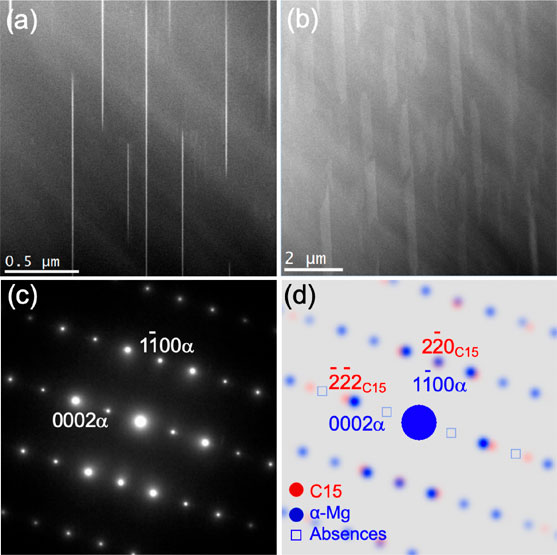
(a) and (b) show HAADF-STEM images of a C15 compound precipitated in the α-Mg grain of the Mg–5Al–3Ca alloy by water-cooling heat-treatment (HT/WC), which were projected along the zone axis $[11\bar{2}0]$α and tilted from the zone axis by 25° on the axis $[1\bar{1}00]$α, respectively. (c) and (d) are a SAED and a simulated diffraction pattern of the C15 compound precipitated in the α-Mg grain of the alloy, respectively.
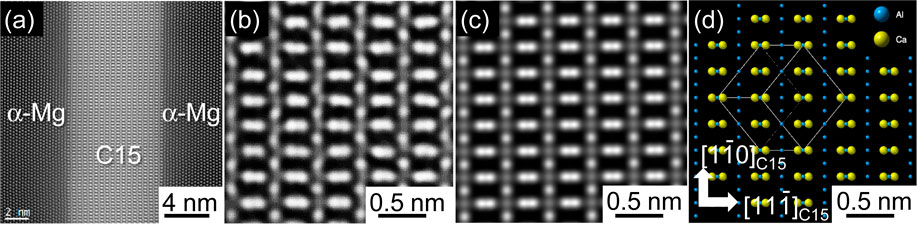
(a) HAADF-STEM image of the C15 compound precipitated in the α-Mg matrix of the Mg–5Al–3Ca alloy by water-cooling heat-treatment (HT/WC). (b) Magnified image of the C15 compound in (a). (c) Simulated HAADF-STEM image of the C15 compound. The multislice simulation was conducted using the model structure (d).
Generally, added solute atoms always have an adverse influence on the thermal conductivity because the solute atoms could induce the lattice distortion of the α-Mg matrix, change the lattice parameter ratio c/a, and shorten the mean free path of electrons and phonons by substituting Mg atoms.4–8) Figure 10 shows the relationship between the solute concentration of Al and Ca elements in the α-Mg matrix and the thermal conductivity of the AC and the HT/WC Mg–(x + 2)Al–xCa alloys. The thermal conductivity increased as the solute concentration in the α-Mg matrix decreased. The thermal conductivity of the solute element-free α-Mg in these Mg–Al–Ca alloys is estimated to be 137 Wm−1K−1, which is smaller by 19 Wm−1K−1 than that of pure α-Mg (156 Wm−1K−1).2) High thermal conductivity of 110 Wm−1K−1 or higher was obtained at less than 1.35 at% of the solute concentration. The purification of the α-Mg matrix seems to be due to the precipitation of the C15 (Al2Ca) compound by the heat treatment.
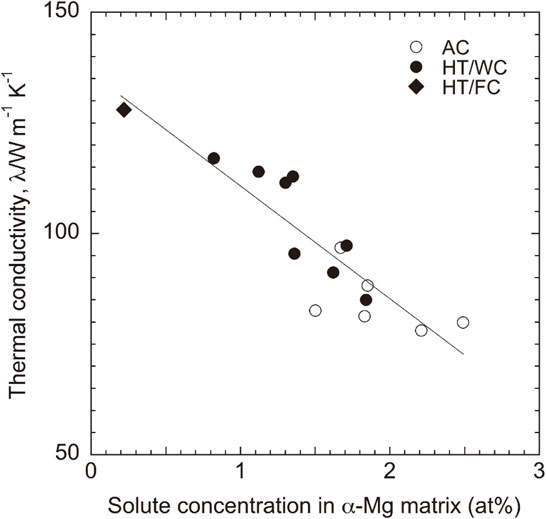
The relationship between the solute concentration of Al and Ca elements and the thermal conductivity of the Mg–(x + 2)Al–xCa alloys in as-cast (AC) and heat-treated (HT/WC and HT/FC) states.
Slower cooling after the heat treatment may improve the thermal conductivity because of promoting the purification of the α-Mg matrix by the acceleration of the C15 precipitation. Therefore, we tried to measure the thermal conductivity of the Mg–5Al–3Ca alloy which was cooled slowly in a furnace after the heat treatment (HT/FC). The concentration of the Al and Ca solute elements in the α-Mg matrix for the Mg–5Al–3Ca alloy was decreased from 1.11 to 0.22 at% by the HT/FC treatment as compared with the HT/WC treatment, resulting in a high thermal conductivity of 127 Wm−1K−1 as shown in Fig. 2 and Table 1.
4.1.2 Effect of C15 precipitation on thermal conductivityThe mixing enthalpy of Mg–Al, Mg–Ca and Al–Ca pairs are −2, −6 and −20 kJ mol−1, respectively.32) The mixing enthalpy of the Al–Ca pair is negatively much larger than that of the Mg–Al and Mg–Ca pairs. Accordingly, the Al and Ca elements, which are dissolved in α-Mg matrix, are easy to combine as compared with the Mg–Al and Mg–Ca pairs, resulting in the acceleration of the C15 precipitation brought about in α-Mg matrix by heat treatment. The C15 precipitation seems to play an important role in the improvement of thermal conductivity due to the purification of the α-Mg matrix.
It has been reported that the incoherent interface between the precipitates and the α-Mg matrix produced relatively minor lattice distortion, resulting in higher thermal conductivity than a coherent interface.4,33) Figures 11(a) and 11(b) show ABF-STEM images of the C15 compound precipitated in the α-Mg matrix of the HT/WC Mg–5Al–3Ca alloy. The advantages of ABF-STEM imaging are (i) higher intensity for light elements, (ii) strain-sensitive diffraction contrast, and (iii) no contrast reversal; therefore, these features are better suited for observing the precipitation structure of the alloy than those of HAADF or LAADF-STEM imaging.34) These images show the diffraction contrast of a ladder-like shape. The contrast means that periodic strain field runs along the interface between the C15 compound and the α-Mg matrix. The strain contrast of the left and the right sides of the C15 compound is not coincident, and the position is shifted with antiphase. The atomic resolution image and its Bragg filtered image using $1\bar{1}00$α, $2\bar{2}0$C15, $\bar{1}100$α and $\bar{2}20$C15 spots shown in Figs. 11(c) and 11(d), respectively, show that the strain contrast corresponds to the coherent region of the interface, while the lattice plane discontinuity in the left and the right sides are 14.0 ± 0.9 nm and 14.2 ± 0.3 nm, respectively. Thus, the interface is incoherent by the gross, but is partially coherent in a cyclic way with antiphase manner in both sides. This structural feature suggests that the local strain energy should be relaxed. It has also been reported that the reduction in thermal conductivity caused by the second phase in the α-Mg matrix is approximately several orders smaller than that by the solute atoms.4,10,35,36) Moreover, the Al and Ca elements constituting the C15 (Al2Ca) compound have a high thermal conductivity of 237 and 201 Wm−1K−1, respectively, which is higher than that of pure Mg (156 Wm−1K−1).37) Accordingly, the C15 precipitates themselves seem to have a weak adverse effect on the thermal conductivity.
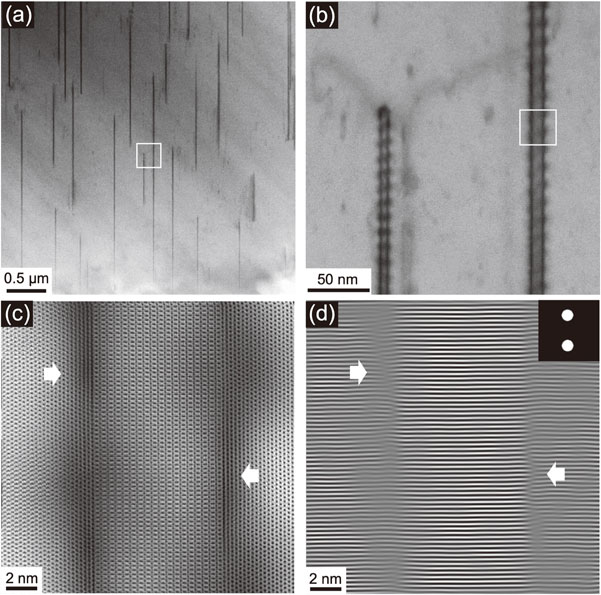
(a) ABF-STEM images of the C15 compound precipitated in the α-Mg matrix of the Mg–5Al–3Ca alloy by water-cooling heat-treatment (HT/WC). (b) Magnified image of (a). (c) Atomic resolution image of a precipitate. (d) Bragg filtered image of (c) using $1\bar{1}00$α, $2\bar{2}0$C15, $\bar{1}100$α and $\bar{2}20$C15 spots. The inserted arrows point out the incoherent regions along the interface.
The eutectic lamellar structure of the Mg–(x + 2)Al–xCa alloys with high thermal conductivity above 110 Wm−1K−1 in the HT/WC state, which consisted of α-Mg and C36/C14 compounds, occupied a large area fraction ranging from 18 to 50% estimated from the SEM micrographs of the AC alloys, as shown in Fig. 5. Figure 12 shows the relationship between the thermal conductivity (λ) and eutectic area fraction (Af) of the Mg–(x + 2)Al–xCa alloys in the AC and the HT/WC states. The reduction rate in thermal conductivity (λ/Af) was estimated to be roughly 0.26 and 0.51 Wm−1K−1 for the AC and the HT/WC states, respectively. This difference seems to be caused by the morphology of the C36/C14 compounds, the solute concentration of Al and Ca elements and the C15 precipitates in the α-Mg matrix. As shown in Fig. 10, the 19 Wm−1K−1 difference in thermal conductivity between the Mg–Al–Ca alloys with solute element-free α-Mg and the pure Mg metal, which is considered to be caused by the C36/C14 compounds, is not particularly large. Moreover, the thermal conductivity of the Mg–(x + 2)Al–xCa alloys with an Af of 100% was estimated to be approximately 69 and 81 Wm−1K−1 in the AC and the HT/WC states, respectively, which seems to be relatively high. Accordingly, the C36 and C14 compounds may have a weak adverse effect on the thermal conductivity.
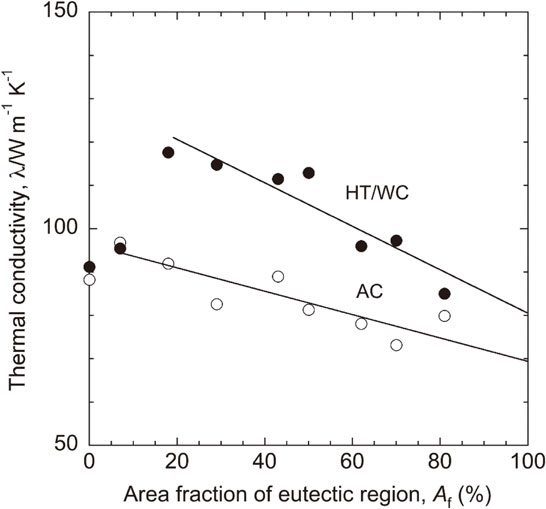
Relationship between the thermal conductivity (λ) and the area fraction of the eutectic region (Af) of the Mg–(x + 2)Al–xCa alloys in as-cast (AC) and water-cooling heat-treated (HT/WC) states.
Figure 13 shows the thermal conductivity of the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex and HT/FC+Ex). For example, the thermal conductivity of the HT/WC+Ex Mg–4Al–2Ca, Mg–5Al–3Ca, Mg–6Al–4Ca alloys and the HT/FC+Ex Mg–5Al–3Ca alloy were 113, 113, 112 and 118 Wm−1K−1, respectively. The hot extrusion improved the mechanical strength but brought about a reduction of 1 to 9 Wm−1K−1 in thermal conductivity. This reduction in the thermal conductivity by the hot extrusion seems to be not so large. This reduction is probably caused not only by the grain boundary and lattice defects but also by the redissolving of the Al and Ca elements into the α-Mg matrix due to heat generation during the extrusion.38)

Thermal conductivity of the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex and HT/FC+Ex).
Figure 14 shows the size of the α-Mg grains and the C36/C14 dispersoids of the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex). The particle size of the C36/C14 dispersoids was approximately 1.5 µm, which did not depend on the alloy composition. It is well known that α-Mg grains become fine by dynamic recrystallization during hot extrusion. The α-Mg grain size of the Ca-free alloy, which consisted of single α-Mg phase, was approximately 20 µm. However, in the alloys containing Ca above 1 at%, the α-Mg grains were drastically refined to below 4.6 µm. Especially, the extruded Mg–(x + 2)Al–xCa alloys with Ca element of 3 at% or larger had a very fine grain size of around 1 µm. The fine α-Mg grains were considered to be due to the suppression of grain growth by the fine C36/C14 dispersoids or the fine C15 precipitates. Nomoto et al. have reported that in a die-cast Mg–4.6Al–0.9Ca (at%) alloy the C15 precipitation, which shows an age-hardening by the heat treatment at 423 to 573 K, exhibits a weak or no age-hardening by the heat treatment at 623 K.39) It is, therefore, considered that the C15 precipitates of the HT/WC+Ex and HT/FC+Ex Mg–(x + 2)Al–xCa alloys have no effect on the improvement of tensile yield strength because the C15 precipitation temperature is 673 K. Figure 15 shows the relationship between the tensile yield strength and the area fraction of the C36/C14 compounds for the HT/WC+Ex Mg–(x + 2)Al–xCa alloys. It is clear that the tensile yield strength increased as the quantity of the C36/C14 compounds increased. The fine α-Mg grains and the homogeneous dispersion of the fine compounds present in a large quantity seems to bring out the high strength and reasonable elongation. It is expected that optimization of the extrusion conditions such as the extrusion temperature, the extrusion ratio and the ram speed may improve the mechanical properties, because mechanical properties of extrudes are strongly affected by the extrusion conditions.38) The HT/FC+Ex Mg–5Al–3Ca alloy prepared by extrusion of the HT/FC billet exhibited high mechanical properties, of which the tensile yield strength and elongation were 337 MPa and 5.2%, respectively, and a high thermal conductivity of 118 Wm−1K−1, as shown in Figs. 4 and 13, and Table 1.
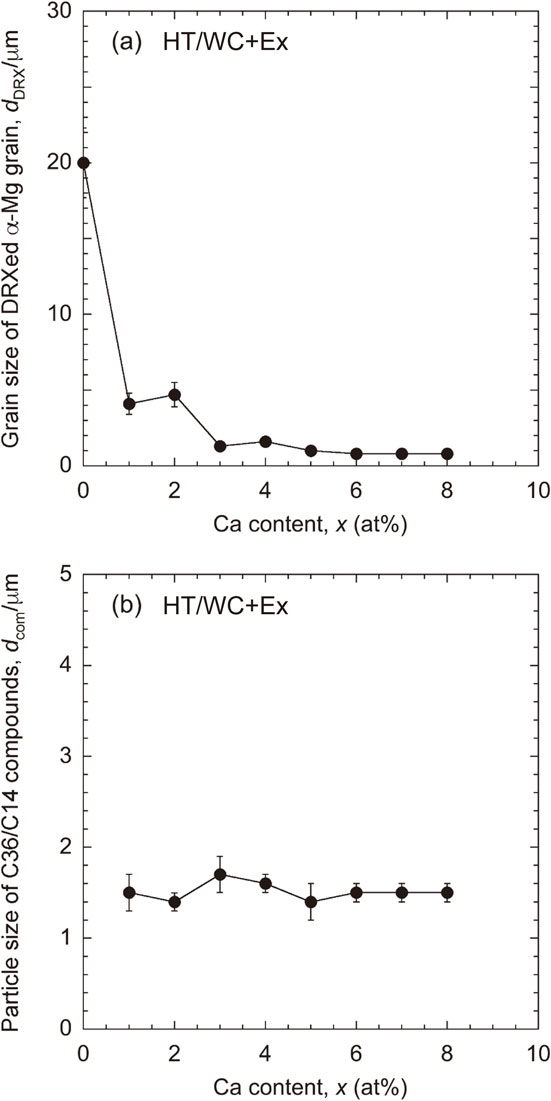
(a) The size of the α-Mg grains. (b) The size of the C36/C14 compounds of the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex).

Relationship between the tensile yield strength and the area fraction of the C36/C14 compounds for the extruded Mg–(x + 2)Al–xCa alloys (HT/WC+Ex).
The definition of non-flammability is often vague. The descriptions of “flame-resistance”, “incombustibility” and “non-flammability” have been ambiguously used for Mg alloys having improved ignition temperature. No Mg alloys exhibiting a high ignition temperature around the boiling point of pure Mg existed until the development of a Mg–10Al–5Ca (at%) alloy.25,26) On the other hand, in 2015, the U.S. Federal Aviation Administration (FAA) has standardized the combustion test of Mg alloys, in which the flame temperature of the oil burner is 1700°F ± 100°F (1200 K ± 38 K).16) Mg alloys having a high ignition temperature above 1238 K never burn in the FAA combustion test. Consequently, there may be two definitions in the critical ignition temperature between non-flammability and flame-resistance: one is the boiling temperature of pure Mg (1364 K), and the other is the maximum flame temperature of the oil burner in the FAA combustion test (1238 K). In this study, the latter definition is adopted. According to this definition, the Mg–(x + 2)Al–xCa alloys with Ca above 2.5 at% exhibited non-flammability. Inoue et al. have reported that Yb and Ca elements have a significant effect for improving the ignition temperature in Mg–1X (at%) binary alloys and discussed a mechanism for improving the ignition temperature from the viewpoint of oxide film formation on the surface.11) The oxide films formed on the Mg–1X alloys are divided into three main types of oxides (MgO, XmOn, and XmOn/MgO) and two subgroups (thermally grown and thermal barrier). The adding Yb or Ca element forms a thermal-barrier-type XmOn film that is thin and dense, resulting in an increased ignition temperature. They have also reported that a trace addition of 0.1 at% Yb, 1.0 at% Ca or 0.03 at% (100 mass ppm) Be improved the ignition temperature up to 1280 K or higher for a LPSO-type Mg–1Zn–2Y alloy having an ignition temperature of approximately 1150 K.11,15) In this study, the Be-addition to an Mg–4Al–2Ca alloy that is not non-flammable has been examined. The trace addition of 0.03 at% Be improved the ignition temperature from 1236 K to approximately 1400 K, resulting in non-flammability, as shown in Fig. 3. The Be element, which is enriched in the surface oxide film, seems to suppress the diffusion of oxygen, resulting in improvement of ignition temperature. It is expected that a trace addition of 0.1 at% Yb improves the ignition temperature of the Mg–4Al–2Ca alloy, as potently as the Be-addition. Since the thermal conductivity and tensile yield strength of the extruded Mg–4Al–2Ca–0.03Be alloy (HT/WC+Ex) were 119 Wm−1K−1 and 318 MPa, respectively, no deterioration in the thermal conductivity and tensile yield strength brought about by the Be-addition was identified.
4.4 Position of the developed Mg–Al–Ca alloys among thermal conductivity wrought Mg alloysFigure 16 compares the thermal conductivity and tensile yield strength for various wrought Mg alloys having a high thermal conductivity above 100 Wm−1K−1.4,40–42) The extruded Mg–4.5Al–2.5Ca, Mg–5Al–3Ca, Mg–6Al–4Ca and Mg–4Al–2Ca–0.03Be alloys (HT/WC+Ex and HT/FC+Ex), which have non-flammability, show the best balance between thermal conductivity and mechanical strength among various wrought Mg alloys with high thermal conductivity, such as Mg–Zn–Mn, Mg–Zn–Zr, Mg–Zn–Cu–Zr, Mg–Mn, Mg–Mn–Ce and Mg–Zn–Ca–Zr alloys. The ignition temperature of these high thermal conductivity wrought Mg alloys has never been reported. It has been reported that non-reactive elements such as Zn, Mn and Cu have no effect on ignition temperature, and that Mg–1 at% X binary alloys with X of reactive Ca or Ce exhibit an improved ignition temperature of 1200 and 948 K, respectively.11) These alloys, therefore, seem to be flammable, because they contain no reactive elements that could improve the ignition temperature, or because they contain only a small amount of the reactive Ca (0.3 at%) or Ce (0.3 at%) element, which is insufficient to improve the ignition temperature. Therefore, it is clear that the extruded Mg–4.5Al–2.5Ca, Mg–5Al–3Ca, Mg–6Al–4Ca and Mg–4Al–2Ca–0.03Be alloys (HT/WC+Ex and HT/FC+Ex) developed in this study simultaneously achieved for the first time the combined properties of high thermal conductivity, high mechanical strength and non-flammability.
High thermal conductivity, high mechanical strength and non-flammability have successfully been achieved simultaneously for the first time in the new Mg–4.5Al–2.5Ca, Mg–5Al–3Ca, Mg–6Al–4Ca and Mg–4Al–2Ca–0.03Be (at%) wrought alloys. Their thermal conductivity, tensile yield strength and ignition temperature were 111 to 119 Wm−1K−1, 318 to 363 MPa and 1343 to 1408 K, respectively. The significant results are listed below.
This work was partially supported by JSPS KAKENHI for Scientific Research A (JP20H00312), JSPS KAKENHI for Scientific Research C (21K04693) and the Light Metal Educational Foundation, Inc.