2022 年 63 巻 3 号 p. 379-388
2022 年 63 巻 3 号 p. 379-388
Preparation of a novel adsorbent, mainly composed of Si–(Al, Fe)–Ca mixed hydrous oxides, from a mixture of coal fly ash and quicklime (CaO) was attempted. Coal fly ash was mixed with NaOH powder and heated to 600°C for 6 h in an electric furnace, and after cooling to room temperature, the produced fused coal ash was mixed with quicklime. The mixture was then stirred in distilled water at room temperature for one day to prepare the adsorbent. With increase in CaO addition to the fused fly ash, the adsorbent changed from an amorphous material to a mixture of calcite [CaCO3] and amorphous phases, then a mixture of calcite and hydrocalumite [Ca4Al2O8(CO3)·11H2O], and finally that of calcite, hydrocalumite, and portlandite [Ca(OH)2]. The removal ability of the adsorbent for NH4+ is almost the same and that for Pb2+ and PO43− increases with increasing CaO addition. The removal of NH4+, PO43−, and Pb2+ depends on the pH of the solution. The adsorbent containing hydrocalumite without portlandite indicates good ability for multifunctional removal in neutral solution and fixation. Fly ash contents, Si and Al, in the adsorbent contribute to NH4+ removal by ion exchange of Si–Al amorphous gels, PO43− removal by synthesis of hydrocalumite to form hydroxyapatite, and Pb2+ removal by formation of PbSi2O5·1.6H2O. The kinetics of the removal of NH4+, PO43−, and Pb2+ using this adsorbent follows the pseudo-second-order kinetics model rather than the pseudo-first-order kinetics model, and the removal of Pb2+ is faster than that of NH4+ and PO43−. These results indicate that a novel adsorbent with removal abilities for NH4+, Pb2+, and PO43− can be prepared from coal fly ash and quicklime, and suggests a new recycling method for industrial wastes.

Recently, atomic power, coal, and natural gas have been considered as substitute energy sources for petroleum. Compared with other energy sources, coal as an energy source has an advantage in terms of abundant deposits. Coal-burning power production considered to 29.9% of global power production in 2011 and it is predicted to reach almost 46% by 2030.1) In 2012, the percentage of electric power generation in coal power plants in Japan occupied approximately 23.4% of the overall electric power.2) Due to an increase in the coal demand, the discharge amount of coal ash is also estimated to have increased. According to the recycling laws in Japan, coal ash derived from thermal power plants is especially designated as a specified by-product, and its effective usage is strongly required. Recently, the reuse and recycling of coal ash have been attempted aggressively, and the percentage and amount of effective usage in 2014 reached approximately 98% and 9.4 million ton/year, respectively. However, more than 200000 ton/year of coal ash must still be deposited for treatment in landfills. Furthermore, since the Great East Japan Earthquake on March 11, 2011, the focus of energy production in Japan has shifted from nuclear power towards coal-generated power. Prior to the earthquake, the fly ash generated at coal-fired power plants was mostly wasted. However, it is expected that more fly ash will be generated in future. In future, the lack of landfill space is anticipated in Japan, hence it is required to develop new recycling technologies for coal ash, by which large amounts of coal ash can be consumed.3–6)
Surface water pollution, which is rapidly increasing with industrialization and urbanization, has become a global environmental problem. The contamination of water by two types of inorganic ions is causing severe problems. These are heavy metal ions and ions such as ammonium and phosphate, which cause eutrophication.
The wastewater discharged by industries that process ores and concentrates of non-ferrous metals is usually polluted with heavy metal ions such as cadmium (Cd2+), lead (Pb2+), nickel (Ni2+), copper (Cu2+), and zinc (Zn2+).7) Several industries involved in metal finishing, mining and mineral processing, coal mining and oil refining, have faced problems associated with heavy metal contamination of process and runoff waters. Therefore, the development of new approaches and technologies is needed to assist in both the removal and recovery of valuable metals from processes and wastewater.8)
Usually, rural municipal wastewater consists of a combination of domestic and commercial wastewaters. The commonly present pollutant parameters are biological oxygen demand (BOD), suspended solids, pathogens, ammonium, nitrate–nitrites, organic-nitrogen, total Kjeldahl nitrogen (TKN), inorganic phosphorus, and organic phosphorus.9) Trace metals and organic pollutant molecules may also be present. Many stock farms are located in rural areas and livestock wastewaters are not always treated adequately. In addition, urea fertilizers and livestock manure are often spread onto the cropped fields. Therefore, intensive stock farming represents a potential source of contamination of groundwater in these areas.
Greater focus is being placed on improving the quality of wastewater being released into surface waters. For small rural municipalities with lower tax bases and limited sources, in order to adopt wastewater treatment technologies, the purifying techniques must have low cost, require minimal maintenance, and be easily applicable. Therefore, low cost adsorbents have been produced from raw materials such as industrial and agricultural wastes,10–15) because recycling and re-use of waste have energy efficient, environmentally friendly, and cost-effective advantages.
In this study, synthesis of a mixed metal hydroxide ash-derived adsorbent from coal fly ash and quicklime, which can be used as a water purification agent, was attempted. Ternary mixed metal hydroxides are composed of three different group elements, i.e., the elements with cationic adsorption properties (Mg, Ca, Fe(II), etc.), those with anionic properties (Si, etc.), and the elements with amphoteric adsorption property (Al, Fe(III), etc.). Such hydroxides have indicated multifunctional removal ability for application in wastewater treatment.16–23) It is also reported that calcined paper sludge including amorphous Ca–Al–Si oxides indicates multifunctional sorption ability for Ni2+, PO43− and NH4+,24) and a calcium aluminosilicate hydrate (CASH) sample was prepared from paper sludge ash, silica, and calcia under hydrothermal condition for the removal of ammonium and phosphate.25) There exists a possibility of synthesis of a mixed metal hydroxide adsorbent with multifunctional removal ability from Si, Al, and Fe content in coal fly ash and Ca content in quicklime (CaO) under mild condition. However, to the best of our knowledge, no studies have yet been conducted on the synthesis of mixed metal hydroxide adsorbents from coal fly ash with quicklime addition for water purification applications. In this study, coal fly ash was treated by alkali fusion. Subsequently, the fused ash was converted to a mixed metal hydroxide adsorbent in distilled water. CaO was added under mild condition. The effect of CaO addition on the synthesis of the adsorbent with multifunctional removal ability from coal fly ash via alkali fusion was investigated. The removal ability of the adsorbent for NH4+, PO43−, and Pb2+ was also examined. Furthermore, the elution test of the adsorbent after the removal test was investigated for application to water purification as a disposable eco-friendly adsorbent.
Coal fly ash was supplied from one of the power plants in Japan. The chemical and mineralogical compositions of the ash, determined using X-ray fluorescence spectrometry (XRF; Epsilon1, PANalytical) for measuring elemental composition from sodium (Na) to a americium (Am) and powder X-ray diffraction (XRD; Rint-2200U/PC-LH, Rigaku) with monochromated CuKα radiation in the 2θ range of 5–60°, respectively, are shown in Table 1 and Fig. 1, respectively. It is noted that all element contents in chemical composition are represent as oxides after calculation of oxide conversion. The ash consisted mainly of SiO2 (53.0%), Al2O3 (19.8%), and Fe2O3 (12.3%), and some low-concentration impurities such as CaO, MgO, K2O, P2O5, and TiO2. The main mineral phases in the ash were quartz [SiO2], mullite [Si2Al6O13], and amorphous phases, leading to the broad hump between 20–30°. The contents of Pb and Ni in fly ash are 17.4 µg/g and 8.3 µg/g, respectively. They would be mainly included in amorphous glass phases, mainly composed of SiO2–Al2O3–CaO together with minor elements, such as MgO, K2O, P2O5 and so on. It may be noted that the specific surface area of the ash was 1.4 m2·g−1.

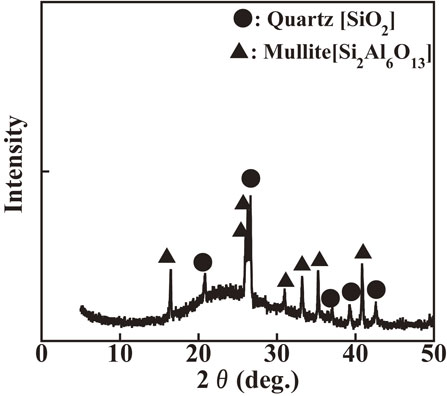
XRD patterns of coal fly ash.
Coal fly ash (10 g) was mixed with 16 g of NaOH powder (Wako, purity 97%), and ground to obtain a homogeneous mixture. This mixture was heated for 6 h at 600°C in air in a nickel crucible. The resultant material was cooled to room temperature and ground again to obtain fused ash. Quicklime (CaO) (Wako, purity 98%) was mixed with the fused ash in the ratio of CaO/fused ash = 0–2 g·g−1, and 5 g of the mixture was added to 20 mL of distilled water. After stirring with a magnetic stirrer for 24 h, the solid was separated by filtration, washed with distilled water until Na+ was not detected, dried at 60°C overnight in a drying oven, and sieved under 250 µm to obtain the adsorbent. The mineralogical phases and chemical compositions of the adsorbents were examined using XRD and XRF, respectively, and the concentrations of Si, Al, Ca, and Fe in the filtrate were determined using inductively coupled plasma atomic emission spectroscopy (ICP-AES; SPS4000, Seiko). The change of the mineralogical phases in the adsorbent was represented using the intensities of their major XRD peaks at the given diffraction faces as follows: calcite (1 0 4), portlandite (0 1 1), and hydrocalumite (0 0 2). The specific surface areas of raw ash and the adsorbents were measured by the Brunauer–Emmett–Teller (BET) method (Macsorb Model-12, Mountech).
2.3 Removal testBatchwise removal tests were carried out on the raw ash and adsorbents for NH4+, PO43−, and Pb2+. The solution of Pb2+ with the concentration of 10 mM was prepared with Pb(NO3)2, assuming an industrial wastewater, and the solutions of both NH4+ and PO43− with the concentration of 10 mM were prepared with NH4H2PO4, assuming a eutrophication water including both NH4+ and PO43−. Then, we added 0.1 gram of the sample to 20 mL of Pb(NO3)2 or NH4H2PO4 aqueous solution in 50 mL of the tube, and the tube was shaken for 18 h with a reciprocal shaker. After shaking at room temperature, the suspension was centrifuged to collect the supernatant. The Pb2+ concentration of the supernatant was analyzed by ICP. The NH4+ concentration was determined by the thymol blue method, and the PO43− concentration was assessed by the molybdenum-blue method. The removal percentages (R (%)) of Pb2+, NH4+, and PO43− were calculated from the differences between the initial and final concentrations in the solution using eq. (1);
| \begin{equation} R = \frac{(C_{0} - C)}{C_{0}} \times 100 \end{equation} | (1) |
The effects of initial concentration, pH, and reaction time on the removal behavior of the adsorbent for NH4+, PO43−, and Pb2+ were examined.
To evaluate the effect of the initial concentration of NH4+, PO43− and Pb2+ on the removal, the adsorbent (50 mg) was added to 20 mL of 0–10 mM NH4H2PO4 solution or 0–10 mM Pb(NO3)2 solution in a 50 mL tube, and the tube was shaken with the shaker for 3 h. After shaking, the suspension was centrifuged to collect the supernatant. The pH of the supernatant was measured with a pH meter (D-53, Horiba), and the concentrations of NH4+, PO43−, and Pb2+ were measured to calculate the removal amount (q (mmol·g−1)) using eq. (2);
| \begin{equation} q = \frac{(C_{0} - C)\cdot V}{w} \end{equation} | (2) |
To evaluate the effect of the pH of the solution, the adsorbent (0.05 g) was added to 20 mL of 10 mM NH4H2PO4 solution or 10 mM Pb(NO3)2 solution in a 50 mL tube, and the tube was shaken with the shaker for 2 h. After shaking, the suspension was centrifuged to collect the supernatant. The pH of the supernatant was measured with a pH meter, and the concentrations of NH4+, PO43−, and Pb2+ were measured to calculate the removal ratio (R (%)).
To evaluate the effect of the reaction time on the removal, 0.1 g of the adsorbent was added to 200 mL of 1 mM NH4H2PO4 solution or 1 mM Pb(NO3)2 solution, and then stirred with a magnetic stirrer. During stirring, the pH of the solution was maintained at 4, 7, and 10 (NH4H2PO4 solution) or 11 (Pb(NO3)2 solution) using HCl dropwise. During stirring, 2 mL aliquots of each slurry were collected at various times, and then filtrated to measure the concentrations of NH4+, PO43−, and Pb2+ in the filtrate to calculate the removal amount (q (mmol·g−1)) at various times. After stirring, the solid was collected by filtration to analyze the mineralogical phases in the adsorbent using XRD after the removal reaction. It is noted that good correlation of measured Pb2+, NH4+ and PO43− in between filtrate and supernatant was confirmed.
2.5 Elution testElution tests were conducted on the raw ash and the adsorbents for Ni2+, which is one of the representative hazardous impurities in coal fly ash, and those on the adsorbent after the removal test for NH4+, PO43−, and Pb2+. The sample (50 mg) was added to 20 mL of distilled water in a 50 mL tube, and the tube was shaken for 1 h with a reciprocal shaker. After shaking at room temperature, the suspension was centrifuged to collect the supernatant. The concentrations of Ni2+ and Pb2+ in the supernatant were analyzed by ICP, the NH4+ concentration was determined by the thymol blue method, and the PO43− concentration was assessed by the molybdenum-blue method to calculate the soluble amounts in the samples (µmol·g−1).
The chemical compositions of the adsorbents synthesized from coal fly ash with various additions of CaO are shown in Table 1. It may be noted that the CaO addition is characterized according to the weight ratio of CaO to fused fly ash (g·g−1). Regarding the main contents of fly ash, the adsorbent synthesized at 0 g·g−1 had a lower content of Si and higher content of Al, Ca, and Fe than raw ash. With increase in the CaO addition, the Ca content of the adsorbent increased, while the content of Si, Al, and Fe decreased.
The XRD patterns of the adsorbent from coal fly ash with various additions of CaO are shown in Fig. 2. Without CaO addition, the adsorbent has no crystalline phases and amorphous phases, thus explaining the broad hump between 20–30° (Fig. 2(a)). It implies that the crystalline phases, quartz and mullite in raw ash, can be completely decomposed by alkali fusion to dissolve into the solution to form amorphous material. With CaO addition, calcite [CaCO3], hydrocalumite [Ca4Al2O6(CO3)·11H2O], and portlandite [Ca(OH)2] are formed in the adsorbent. It is noted that CO32− was supplied from atmosphere by dissolving CO2 into alkali solution during the adsorbent preparation process. With increasing CaO addition, calcite appears in the adsorbent (Fig. 2(b)), then hydrocalumite appears (Fig. 2(c)), and finally a mixture of calcite, hydrocalumite, and portlandite is formed (Fig. 2(d)). It is noted that silicate mineral phases do not appear in the adsorbent, implying that Si content in the solid phase is present as amorphous phases. The specific surface areas of the adsorbent with CaO addition lower than 0.3 g·g−1 are 5–10 m2·g−1, while those of the adsorbent with CaO addition higher than 0.4 g·g−1 are approximately 20 m2·g−1. This suggests that the increase in the specific surface area is due to the formation of the new crystalline phases, hydrocalumite and portlandite.

XRD patterns of the adsorbents from coal fly ash with the addition of CaO. (a) 0 g·g−1, (b) 0.2 g·g−1, (c) 0.4 g·g−1, (d) 2.0 g·g−1.
The concentrations of Si, Al, Ca, and Fe in the solution after synthesis, and the intensities of the mineral phases in the adsorbent, as a function of CaO addition are shown in Fig. 3. Without CaO addition, the concentrations of Si, Al, Ca, and Fe are 4569 mg·L−1, 602 mg·L−1, 0 mg·L−1, and 3 mg·L−1, respectively, and an amorphous gel is formed, as shown in Fig. 2(a). With increasing CaO addition from 0 to 0.2 g·g−1, the Si concentration decreases, Al concentration increases, the Ca and Fe concentration is almost zero, and the intensities of calcite increase. It can be considered that a part of the additional CaO is formed as calcite by reaction with carbonate ions in alkali solution dissolved from atmosphere (CaO + CO2 → CaCO3), and a part of the CaO reacts with Si and Fe to form Ca–Si–Fe hydroxide gel. When formed without CaO addition, Al remains in the solution and does not form Si–Al gel, because the reaction between Ca and Si is superior to that between Si and Al.26) For CaO addition of 0.3–0.5 g·g−1, the Si, Ca, and Fe concentrations are almost zero, and the Al concentration decreases. In the adsorbent, the intensity of hydrocalumite increases, and that of calcite is almost constant. It can be considered that hydrocalumite is formed by the reaction between CaO and Al in the solution due to the shortage of Si (4CaO + 2Al(OH)4− + CO32− + 9H2O → Ca4Al2O6(CO3)·11H2O + 4OH−). With CaO addition over 0.5 g·g−1, the Si, Al, and Fe concentrations are almost zero. The Ca concentration increases, while the intensities of calcite and hydrocalumite decrease and that of portlandite increases. It can be considered that the Si and Al in fused fly ash completely react with additional CaO, and the unreacted CaO is formed as portlandite in the alkali solution (CaO + H2O → Ca(OH)2).
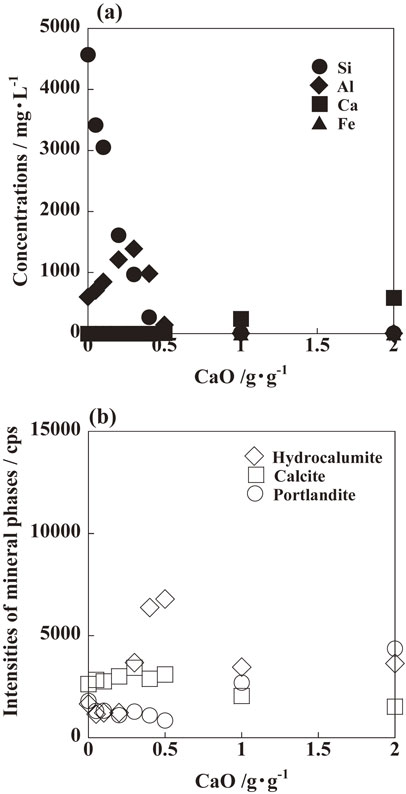
(a) Si and Al concentrations in the solution, and (b) intensities of the mineral phases in the adsorbent after synthesis.
The removal abilities of the adsorbent for NH4+, PO43−, and Pb2+ and the pH of the solution after the removal test as a function of CaO addition are shown in Fig. 4. For NH4+ and PO43− (Fig. 4(a)), the removal abilities of raw ash for NH4+ and PO43− are 6.0% and 6.2%, respectively, and the pH of the solution after the removal test is 6.1. Without CaO addition, the removal abilities of the adsorbent for NH4+ and PO43− are 52.9% and 8.0%, respectively, due to the amorphous gel, and the pH of the solution after the test is 7.8. With increasing CaO addition to 0.3 g·g−1, the pH of the solution increases to 10, and gradually increases to 12 above 0.3 g·g−1 addition, due to the increase in the alkalinity of the adsorbent with the addition of CaO. For NH4+, the removal ability of the product is 50–60% below 0.3 g·g−1 addition; otherwise, it is almost constant at approximately 45%. It can be considered that the Si–Al gel with high cation exchange capacity is converted to Ca–Si–Fe or Ca–Si–Al gel, which have a lower cation exchange capacity than Si–Al gel. For PO43−, the removal ability increases with increasing CaO addition. It can be considered that additional CaO in the adsorbent contributes to the removal of PO43− in the solution. For Pb2+ (Fig. 4(b)), the removal ability of raw ash is 0%, and the pH of the solution after the removal test is 4.9. Without CaO addition, the removal ability of the adsorbent for Pb2+ is 66.2%, and the pH of the solution after the test is 6.7. With CaO addition increasing to 0.2 g·g−1, the pH of the solution and the removal ability for Pb2+ rapidly increase to 11 and 99%, respectively, and are almost constant with CaO addition above 0.2 g·g−1. It can also be considered that additional CaO in the adsorbent contributes to the removal of Pb2+ in the solution.

Removal ability of the adsorbent with various CaO additions, and pH of the solution after the removal test in the (a) NH4H2PO4 solution and (b) Pb(NO3)2 solution.
From these results, it can be inferred that mixed metal hydroxide ash-derived adsorbent with multifunctional removal ability for NH4+, PO43−, and Pb2+ can be synthesized from coal fly ash with the addition of CaO. These are classified into four types, i.e., the adsorbent with CaO addition of 0 g·g−1, 0–0.2 g·g−1, 0.2–0.5 g·g−1 and 0.5–2.0 g·g−1. It is noted that Ni2+ elution from all adsorbents is not detected, while that from raw ash is 0.02 mg·g−1.
3.2 Removal reactionThe removals of NH4+, PO43−, and Pb2+ in aqueous solution with various initial concentrations (0–20 mM) were examined using four typical adsorbents obtained with CaO addition of 0 g·g−1 (Adsorbent-1), 0.2 g·g−1 (Adsorbent-2), 0.4 g·g−1 (Adsorbent-3), and 2.0 g·g−1 (Adsorbent-4).
The isotherms of the adsorbents, coal fly ash, CaCO3, Ca(OH)2, and hydrocalumite, which was synthesized by the method of Wajima,27) for NH4+, PO43−, and Pb2+ are given, and these isotherm data were analyzed using the Langmuir and Freundlich models28,29) as follows:
| \begin{equation} \frac{C_{e}}{q_{e}} = \frac{1}{q_{\text{max}}\cdot K_{L}} + \frac{1}{q_{\text{max}}} \cdot C_{e} \end{equation} | (3) |
| \begin{equation} \ln (q_{e}) = \ln (K_{F}) + \frac{1}{n}\ln (C_{e}) \end{equation} | (4) |
The isotherms of NH4+ using (a) the adsorbent and coal fly ash, and (b) CaCO3, hydrocalumite and Ca(OH)2 are shown in Fig. 5, and the parameters calculated for the Langmuir and Freundlich equations are listed in Table 2. It is noted that the pH of the solution after the removal test is alkaline (9–11). From the R2 values, it follows that the experimental data for Adsorbent-1, Adsorbent-2, and Adsorbent-3 fit the Langmuir model better than the Freundlich model, while that for Adsorbent-4 fits the Freundlich model better than the Langmuir model. Coal fly ash and CaCO3 indicate no adsorption ability for NH4+. The isotherm of Ca(OH)2 is fitted well to the Freundlich equation, while that for hydrocalumite is fitted better to the Langmuir equation. With increasing CaO content in the adsorbent, the adsorbent has the adsorption ability for NH4+ due to the amorphous phase and hydrocalumite, and finally becomes almost the same as Ca(OH)2 due to the significantly higher content of Ca(OH)2 in the adsorbent. Adsorbent-3 has the maximum adsorption capacity (3.22 mmol·g−1), which is higher than sepiolite (0.1 mmol·g−1) and clinoptilolite (1.1 mmol·g−1).30)

Isotherm of NH4+ using (a) the adsorbent and coal fly ash, and (b) CaCO3, hydrocalumite, and Ca(OH)2.

The PO43− isotherms of the adsorbents, coal fly ash, CaCO3, hydrocalumite, and Ca(OH)2 are shown in Fig. 6, and the parameters calculated for the Langmuir and Freundlich equations are listed in Table 3. It is noted that the pHs of the solution after the removal test are in the alkaline range (9–11). From the R2 values, it follows that the experimental data for Adsorbent-1 and Adsorbent-2 fit the Freundlich model better than the Langmuir model, while that for Adsorbent-3 and Adsorbent-4 fit the Langmuir model better than the Freundlich model. Coal fly ash indicates no adsorption ability for PO43−. The isotherm of Ca(OH)2 and hydrocalumite is well fitted to the Langmuir model, while that of CaCO3 is fitted better to the Freundlich model. With increasing CaO content in the adsorbent, the adsorbent has the adsorption ability for PO43− due to the presence of hydrocalumite and portlandite (Ca(OH)2). Adsorbent-3 and Adsorbent-4 show the maximum adsorption capacities, 6.22 mmol·g−1 and 6.78 mmol·g−1, respectively, which is higher than that of calcined paper sludge (2.0 mmol·g−1),13) synthesized La(OH)3 (1.1 mmol·g−1),31) and granular iron hydroxide (5.3 mmol·g−1).32)

Isotherm of PO43− using (a) the adsorbent and coal fly ash, and (b) CaCO3, hydrocalumite, and Ca(OH)2.

The Pb2+ isotherms of the adsorbents, coal fly ash, CaCO3, hydrocalumite, and Ca(OH)2 are shown in Fig. 7, and the parameters calculated for the Langmuir and Freundlich equations are listed in Table 4. It is noted that the pHs of the solution after the removal test are in the alkaline range (8–11). From the R2 values, it follows that the experimental data for Adsorbent-1, Adsorbent-2, and Adsorbent-3 fit the Langmuir model better than the Freundlich model, while that for Adsorbent-4 fits the Freundlich model better than the Langmuir model. Coal fly ash shows considerably less adsorption ability for Pb2+. The isotherm of Ca(OH)2 is well fitted to the Freundlich model, while those of CaCO3 and hydrocalumite are fitted better to the Langmuir model. With increasing CaO content in the adsorbent, the adsorbent has the adsorption ability for Pb2+ due to the amorphous phase, calcite (CaCO3), and hydrocalumite, and finally becomes almost the same as that of Ca(OH)2 due to the considerably higher content of Ca(OH)2 in the adsorbent. This is the same as NH4+ removal. Adsorbent-3 shows the maximum adsorption capacity (3.80 mmol·g−1), which is higher than that of activated carbon (0.65 mmol·g−1) and biochar (3.0 mmol·g−1).33,34)

Isotherm of Pb2+ using (a) the adsorbent and coal fly ash, and (b) CaCO3, hydrocalumite, and Ca(OH)2.

The effects of the pH of the solution on the removal reactions for NH4+, PO43−, and Pb2+ were investigated. The final pHs of the solution after removal using the adsorbents from the solution with various initial pHs are shown in Fig. 8. In both solutions, the pHs of the solution increase from the initial values, and show almost constant final pHs for the solution with initial pH in the range 3–6. The order of the final pHs in both solutions is Adsorbent-4 > Adsorbent-3 > Adsorbent-2 > Adsorbent-1, indicating that the adsorbent becomes more alkaline with increasing CaO content.

Final pH of the solution after removal using the adsorbent from the (a) NH4H2PO4 solution and (b) Pb(NO3)2 solution, with various initial pH values.
The removal of (a) NH4+, (b) PO43−, and (c) Pb2+ from the solution with various pHs using the adsorbents are shown in Fig. 9. For all species, with increasing final pH, the removals of all species increase, and then are almost constant above pH 6–8. The adsorbent has multifunctional removal ability for NH4+, PO43−, and Pb2+ in natural water (pH 6–9).

Removals of (a) NH4+, (b) PO43−, and (c) Pb2+ from the solution with various pHs using the adsorbents.
The adsorbents (0.1 g) were stirred in 200 mL of 1 mM NH4H2PO4 solution or 1 mM Pb(NO3)2 solution while maintaining pH = 7 for 1 h to calculate the adsorption amount, and then the elution amounts of the adsorbents after removal were estimated. The adsorption and elution amounts of the adsorbents are shown in Table 5, and the XRD patterns of the adsorbents after adsorption are shown in Fig. 10. It is noted that the pH of the solution after the elution test for Adsorbent-1, Adorbent-2, and Adsorbent-3 are in the range 7–9 while that for Adsorbent-4 is 11. The amount of NH4+ adsorption using Adsorbent-1 is higher than that using Adsorbent-2, Adsorbent-3, and Adsorbent-4, while the amounts of PO43− using Adsorbent-2, Adsorbent-3, and Adsorbent-4 are higher than that using Adsorbent-1. After adsorption in NH4H2PO4 solution, the peaks of calcite and hydroxyapatite [Ca5(PO4)3(OH)] were confirmed in the spent adsorbent-2 and spent adsorbent-3. Those of calcite, brushite [CaHPO4·2H2O], and portlandite [Ca(OH)2] were confirmed in the spent adsorbent-4, while no peaks were confirmed in the spent adsorbent-1. It can be considered that PO43− removal depends on the formation of hydroxyapatite (5Ca2+ + 3PO43− + OH− → Ca5(PO4)3(OH)). It is not confirmed that a mineral related to NH4+ is formed after the removal test. It can be considered that NH4+ removal depends on the sorption of amorphous Si–Al, Ca–Si, Ca–Si–Al gels in the adsorbent. All adsorbents completely remove Pb2+ at pH 7. After adsorption in Pb(NO3)2 solution, the peaks of calcite, cerussite [PbCO3], and lead bisilicate hydrate [PbSi2O5·1.6H2O] are confirmed in the spent adsorbents. It can be considered that Pb2+ removal depends on the formation of PbSi2O5·1.6H2O by the reaction between soluble silica from amorphous silicate gel in the adsorbent and Pb2+ in the solution (Pb2+ + Si2O52− + 1.6H2O → PbSi2O5·1.6H2O), and PbCO3 precipitation by variation in the pH of the solution from 5 to 7 (Pb2+ + CO32− → PbCO3). Pb2+ is the most important ion for elution, and its elution in Adsorbent-3 is lower than 0.1 µmol·g−1 (below detectable limit), while that of Adsorbent-4 is high (28.3 µmol·g−1). The elution of NH4+ from spent adsorbent-3 is 8.7 µmol·g−1 (6.2%), which is the lowest. The elution of PO43− from spent adsorbent-2 and adsorbent-3 are 54.3 µmol·g−1 (5.6%) and 57.6 µmol·g−1 (5.1%), respectively, while those of spent adsorbent-1 and adsorbent-4 are 4.7 µmol·g−1 (3.4%) and 0.4 µmol·g−1 (<0.1%), respectively, because hydroxyapatite has higher solubility than brushite. An ecofriendly one-way adsorbent must have high removal abilities, allow the release of marginal amounts of nutrients into the water area, and must fix the heavy metals in the adsorbent due to the creation of a biotope environment. From these results, Adsorbent-3 is preferable to use as a one-way eco-friendly adsorbent.


XRD patterns of the adsorbent after adsorption in (a) NH4H2PO4 solution and (b) Pb(NO3)2 solution at pH 7.
The adsorption of Adsorbent-3 for NH4+, PO43−, and Pb2+ during the reaction in the solution at pH 4–11 is shown in Fig. 11. For NH4+ and PO43− (Fig. 11(a), (b)), the adsorption amounts increase at the initial stage for 20 min, and then reach equilibrium. For NH4+ removal, with increasing pH of the solution, the removal increases. For PO43−, regardless of the pH of the solution, the removal after equilibrium is almost the same (80–100%). For Pb2+ (Fig. 11(c)), the adsorption amount rapidly increases initially for five minutes, and then reaches equilibrium. The Pb2+ removals in the solutions with pH 7 and 11 are more than 95%, while that with pH 4 is approximately 20%. These results indicate that the removal reactions are different for NH4+, PO43−, and Pb2+, and the effective removal of all ions can be conducted in the neutral-alkali solution.

Adsorption of (a) NH4+, (b) PO43−, and (c) Pb2+ in the solution during the reaction at pH 4–11.
The XRD patterns of the spent mixed metal hydroxide ash-derived adsorbent-3 after the removal test in the solution with various pHs are shown in Fig. 12. In NH4H2PO4 solution, the peaks of calcite and hydroxyapatite are confirmed in the spent adsorbent after the test using the solution with pH 7 and 10, while no peaks are confirmed in the spent adsorbent after the test using the solution with pH 4. The NH4+ removal depends on the cation sorption of amorphous Si–Al, Ca–Si, or Ca–Si–Al gels in the adsorbent, and decreases in lower pH solution due to the competition between protons (H+) and NH4+ for adsorption sites on the adsorbent. The PO43− removal depends on the formation of hydroxyapatite due to the dissociation states of phosphate ions and supply of OH−. In Pb(NO3)2 solution, the peaks of calcite, cerussite, and lead bisilicate hydrate are confirmed in the spent adsorbent after the test using the solution with pH 7 and 11, while no peaks are confirmed in the spent adsorbent after the test using the solution with pH 4. It can be considered that the formation of PbSi2O5·1.6H2O and PbCO3 occur in the solution at pH 7–11 to remove Pb2+, because increase of pH promotes the dissolution of silica content in the adsorbent and CO2 from atmosphere.

XRD patterns of adsorbent-3 after the removal test in (a) NH4H2PO4 solution and (b) Pb(NO3)2 solution with various pHs.
The kinetics results for NH4+, PO43−, and Pb2+ in the solution at pH 7 in Fig. 11 were analyzed using pseudo-first-order and pseudo-second-order models as:
| \begin{equation} \ln (q_{e} - q_{t}) = \ln (q_{e}) - k_{1}\cdot t \end{equation} | (5) |
| \begin{equation} \frac{t}{q_{t}} = \frac{1}{(k_{2}\cdot q_{e}^{2})} + \frac{t}{q_{e}} \end{equation} | (6) |

Coal fly ash was attempted to convert mixed metal hydroxide ash-derived adsorbents for use as a water purification agent. The ash was treated by alkali fusion, and the mixture of fused fly ash and quicklime (CaO) was stirred in distilled water under mild condition to synthesize the adsorbent with multifunctional removal ability. The adsorbent showed multifunctional removal ability for NH4+, PO43−, and Pb2+. With increasing CaO addition, the removal of PO43− by the adsorbent increases due to the formation of hydroxyapatite by a reaction between Ca content in the adsorbent and PO43− in the solution. The removal of Pb2+ by the adsorbent also increases due to the formation of PbSi2O5·1.6H2O by the reaction between the Si content of amorphous phases in the adsorbent and Pb2+ in the solution and the precipitation of PbCO3 by increasing the pH of the solution. The removal of NH4+ is caused by the content of the amorphous phases in the adsorbent. Fly ash contents, Si and Al, in the adsorbent contribute to NH4+ removal by ion exchange of Si–Al amorphous gels, PO43− removal by synthesis of hydrocalumite to form hydroxyapatite, and Pb2+ removal by formation of PbSi2O5·1.6H2O. The adsorbent containing hydrocalumite and without portlandite has good multifunctional removal ability of NH4+, PO43−, and Pb2+, and the elution of these ions is quite low to facilitate use as a disposable eco-friendly adsorbent.
These results suggest that a novel adsorbent with multifunctional removal ability can be synthesized from coal fly ash and quicklime. Coal fly ash and quicklime are thus, cheap raw materials for producing adsorbents with a high capacity for removing heavy metal, phosphate, and ammonium ions. These materials appear to be suitable for reducing the environmental pollution caused by the presence of these three ionic contaminants in water.
This work was supported by JSPS KAKENHI Grant Number 20K12234.