2022 年 63 巻 3 号 p. 335-342
2022 年 63 巻 3 号 p. 335-342
On free surfaces without crevice structures, micro pits of several micrometers in diameter that are repassivated immediately are called metastable pits. The role of the micro pit as an initiation site of crevice corrosion was examined by comparing the metastable pitting corrosion potential (V′cMS) of SUS304 stainless steel measured on the free surface with the critical potential for crevice corrosion (VCREV). VCREV presented the similar value to V′cMS. It was also revealed that micro pits, as well as SUS304 stainless steel surface, were hard to be repassivated at pH ≤ 2.0, meaning that micro pits can be initiation sites of crevice corrosions. Investigating Cl− concentration and pH dependencies on each potential, the influences of these factors were also examined in terms of the contribution to a crevice corrosion initiation. Cl− ions, which have V′cMS and VCREV less noble, will directly contribute to a crevice corrosion initiation by facilitating micro pit initiations. pH decrease accelerating passive dissolution would promote a crevice corrosion initiation indirectly by increasing Cl− migration into a crevice structure.
This Paper was Originally Published in Zairyo-to-Kankyo 70 (2021) 32–39. The captions of Figs. 4, 7 are slightly modified.
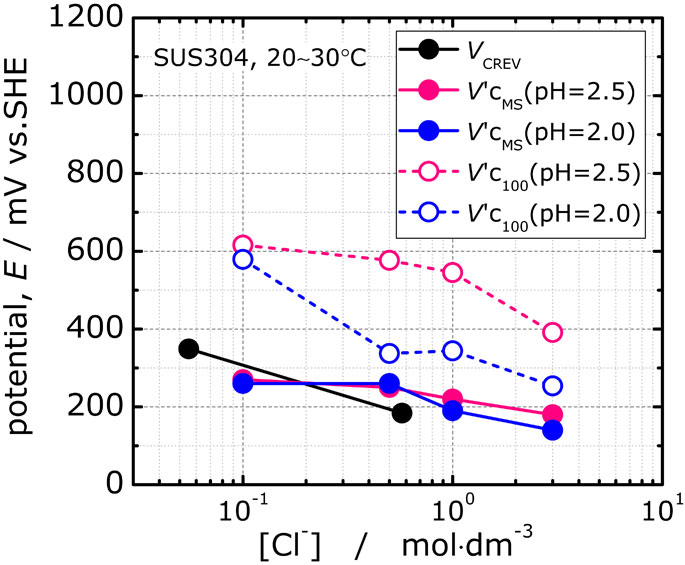
Fig. 13 The relationships between V′cMS, V′c100 and VCREV at estimated Cl− concentration in crevice solution.
Stainless steels are iron-based alloys containing more than 10.5% Cr.1) In many environments, the passive film is formed on the surface of stainless steel, and the corrosion resistance can be maintained. However, when the steel has a crevice structure, the environment in the crevice is affected by (1) loss of dissolved oxygen, (2) lowering of pH due to hydrolysis of metal ions accumulated in the crevice, and (3) inflow of Cl− from outside the crevice into the crevice by electrophoresis, which may destroy the passive film and cause crevice corrosion.2)
Two types of crevice corrosion mechanisms have been proposed: active dissolution type and pitting corrosion type.3) In the active dissolution type crevice corrosion mechanism, the pH in the crevice decreases below the depassivation pH (pHd) due to passive dissolution, i.e., hydrolysis of a small amount of metal ions leached from the passive film, and the passive film is reduced and dissolved.2,3) On the other hand, in the pitting corrosion type crevice corrosion initiation mechanism, it is explained that the initiation of pitting in the crevice is the starting point of crevice corrosion,3) and it is thought that the passive stainless steel is activated by the occurrence of pitting corrosion4) and crevice corrosion progresses.
Although the pHd of SUS304 stainless steel is generally recognized to be 2.0,5) there is an example where it was confirmed that the actual depassivation occurs around 0,6) suggesting that the passive film will not dissolve unless the pH inside the crevice is significantly lowered by passive dissolution. On the other hand, in the pitting corrosion type, micro pits of several micrometers in diameter were observed in the crevice of the stainless steel in which crevice corrosion occurred, and were reported to play an important role in the crevice corrosion.7,8) Such micro pits are called metastable pits because they immediately repassivate on the free surface without crevice structure.7) The stable pits that grow stably on the free surface are considered to be more than 40 to 100 µm in diameter.9) In this paper, stable pits are simply referred to as pits and are distinguished from metastable pits.
In this paper, the role of the micro pit in the crevice corrosion initiation process was investigated because the same pitting corrosion type initiation mechanism can occur in SUS304 stainless steel. We summarized the relationship between the pitting corrosion potential and metastable pitting corrosion potential of SUS304 stainless steel and the critical potential for crevice corrosion initiation, and discussed the role of micro pit as the starting point of crevice corrosion.
The pitting corrosion potential and metastable pitting corrosion potential were measured in aqueous chromium chloride solution,2,10) which is one of the highly corrosive components of the crevice solution and is largely responsible for lowering the pH by hydrolysis reaction. In addition, the dependence on Cl− concentration and pH was investigated in the environment with constant Cl− concentration and pH, and the effect of these factors on the crevice corrosion was discussed.
Commercial SUS304 stainless steel was used in the experiments. The chemical composition of the specimens is shown in Table 1.

In order to clarify the basic electrochemical behavior of stainless steel, free-surface specimens were prepared. The specimens were cut from the product plate to the dimensions of 10w × 15L × 3t mm, and then the entire surface of the specimen was wet-polished to #400 using SiC water-resistant abrasive paper. The lead wires were soldered to the top of the specimen, and the specimen was degreased in acetone using ultrasonic waves for about 10 min, then dried in cold air and sealed with silicone coating (Shin-Etsu Silicone KE45RTV). For those with polarization curves measured, only 0.5 cm2 of the surface area was left and the other surfaces were sealed.
Next, crevice corrosion test specimens were fabricated to investigate the crevice corrosion behavior. A schematic diagram of the specimens is shown in Fig. 1. The test specimens were cut out from the product plate to the dimensions of 20w × 50L × 4t mm, and then the entire surface of the specimen was wet-polished to #400 using SiC water-resistant abrasive paper. Enameled conductors were soldered to the top edge of the specimen, and the soldered area was sealed. The specimens were then immersed in a 30% HNO3 solution at 50°C for 1 h for passivation treatment. Immediately before the test, the test surface (16 mm × 16 mm) was again wet-polished with #400 emery paper, and with the test solution applied to the test surface, the 16w × 16L × 3t mm glass was laminated and fixed using a polycarbonate bolt and nut jig and titanium washers. The tightening torque was set at 0.18 N-m. The specimen and the glass have 6φ holes for fixing the bolt and nut jig.

The schematic illustration of the specimen with crevice.
Aqueous chromium chloride (CrCl3) solution, which has the greatest effect on the decrease in pH, was used to simulate the solution inside the crevice.
CrCl3·6H2O reagent (Kanto Chemical Co., Inc., Cica-Reagent, special grade) and pure water were mixed to obtain [CrCl3] = 0.001, 0.01, 0.03, 0.1, 0.2, 0.4, 0.6, 0.8, 1.0 mol·dm−3. Purified water with a conductivity of less than 2.1 µS/cm was used.
It takes a considerable amount of time for the complex ion reaction in CrCl3 solution to reach equilibrium.10) In order to lower the pH of the solutions sufficiently by the hydrolysis of Cr3+, the solutions were used after more than 500 h from the time of preparation.
2.2.2 Sodium chloride + hydrochloric acid solution and artificial seawaterNaCl and HCl (both Kanto Chemical Co., Inc., Cica-Reagent, special grade) were mixed with pure water, and NaCl and HCl aqueous solutions were adjusted to [Cl−] = 0.1, 0.5, 1.0, and 3.0 mol·dm−3, respectively. Then, NaCl and HCl solutions with the same Cl− concentration were mixed to adjust various pH values.
For the artificial seawater solution, ASTM-artificial seawater (D-1141-52) and diluted artificial seawater by adding pure water and diluted 100 times (chloride ion concentration: 18900, 189 ppm) were prepared.
2.3 Electrochemical measurementsElectrochemical measurements included (1) pitting corrosion potential and metastable pitting corrosion potential measurements due to anodic polarization, (2) critical potential measurements for crevice corrosion initiation, and (3) investigation of whether the corrosion reaction occurred in the active or passive state. The aqueous solution used for these electrochemical measurements was 25 ± 5°C. A silver chloride electrode (Ag/AgCl in saturated KCl) was used as a reference electrode, and the obtained potential was converted to SHE. From the temperature dependence of the silver chloride electrode and SHE,11) the conversion formula to SHE was obtained when the temperature of the aqueous solution was T°C. The conversion equation is shown in eq. (2.1). In this paper, all the obtained potentials are converted to SHE.
| \begin{equation} \text{mV vs. SHE} = \text{mV vs. SSE} + (224 - T) \end{equation} | (2.1) |
CrCl3 solution and NaCl + HCl solution were used for pitting corrosion potential and metastable pitting corrosion potential measurements. The specimens were degassed in the liquid phase with Ar for more than 30 min before the measurement and in the gas phase during the measurement. The specimen (free surface material) was immersed in the aqueous solution and immediately swept from −500 mV in the anodic direction at a rate of 20 mV/min. The purpose of starting at −500 mV, where the cathode current flows, was to remove the film formed on the surface and to obtain the anodic polarization curve from as low a potential as possible, since metastable pitting corrosion might occur at lower potentials.
From the obtained anodic polarization curve, the potential at which pitting corrosion and metastable pitting corrosion first occurred was investigated. The schematic diagram of the anodic polarization curve and the analysis method are shown in Fig. 2. The specific method for determining metastable pitting corrosion is described in detail in Section 3.1. The metastable pitting potential (V′cMS) is the least noble potential at which metastable pitting occurs, and the potential at which stable pitting occurs and the current density reaches 1 A·m−2 (100 µA·cm−2) is the pitting potential (V′c100). The current density at E = 200 mV, which is the potential before the onset of pitting corrosion at any pH and is in the passive region, is defined as ipass, and the maximum current density in the active region is defined as iCRIT.
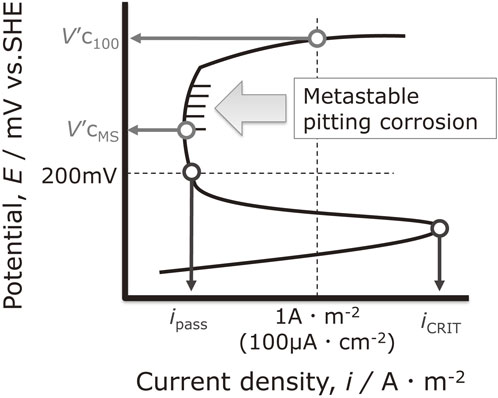
Analysis of anodic polarization curves.
Room temperature artificial seawater open to the atmosphere and 100 times diluted artificial seawater were used to measure the critical potential for crevice corrosion initiation (VCREV). The crevice-giving specimens, whose crevice surfaces were polished to #400 immediately before the tests, were held at various values of constant potential, and the current-time curves were measured. The specimens were immersed in an aqueous solution and the potential was applied when the natural immersion potential showed a value of −56 ± 10 mV. The least noble potential, at which an upward trend in current value was observed and crevice corrosion occurred, was designated as VCREV.
The active and passive states of the specimens were investigated in various concentrations of CrCl3 solution. The specimens were immersed in an aqueous solution during liquid phase degassing with Ar gas for 24 hours, and the spontaneous immersion potential of the specimens (free surface material) was measured. Just before immersion, the immersed surface of the specimen was dry-polished with SiC water-resistant abrasive paper at #400 to remove the film formed on the specimen in the air. After 24 h of immersion, specimens that maintained a redox potential less noble than that of the hydrogen ion in eq. (2.2) were considered to be in the active state, while those that were nobler were considered to be in the passive state.12,13)
| \begin{equation} \text{E} = -59\,\text{mV} \times \text{pH} \end{equation} | (2.2) |
The anodic polarization curves of SUS304 stainless steel specimens in various concentrations of aqueous CrCl3 solutions are shown in Fig. 3. Metastable pitting corrosion was observed in each polarization curve, where a small increase in the current density and a return to the passive current density were repeatedly observed after entering the passive region beyond the active region. The polarization behavior of each CrCl3 solution was observed.

Anodic polarization curves of SUS304 in CrCl3 solutions.
The V′c100 and V′cMS obtained from the polarization curves of each aqueous CrCl3 solution are shown in Fig. 4 along with the pH of the solution. As the concentration of CrCl3 in the aqueous solution increased, both V′c100 and V′cMS tended to become less noble, indicating that pitting corrosion and metastable pitting corrosion in the crevices were more likely to occur.

Pitting potential (V′c100) and metastable pitting potential (V′cMS) of SUS304 in CrCl3 solutions.
As the concentration of CrCl3 increases, the Cl− concentration in the aqueous solution increases and the pH decreases. The same test was conducted in a constant Cl− concentration and pH environment using NaCl + HCl solution. An example of the anodic polarization curves is shown in Fig. 5. Figure 5(a) shows the anodic polarization curves when the Cl− concentration was varied at a constant pH of 2.5, and Fig. 5(b) shows the anodic polarization curves when the pH was varied at a constant [Cl−] = 0.5 mol·dm−3. The V′c100 and V′cMS obtained from each anodic polarization curve are shown in Fig. 6 and Fig. 7, respectively.

Anodic polarization curves of SUS304 in NaCl + HCl solutions. (a) pH = 2.5, (b) [Cl−] = 0.5 mol·dm−3

The effects of (a) chloride ion concentration and (b) pH on pitting potential (V′c100) of SUS304 in NaCl + HCl solutions.

The effects of (a) chloride ion concentration and (b) pH on metastable pitting potential (V′cMS) of SUS304 in NaCl + HCl solutions.
As shown in Fig. 6, V′c100 became less noble as the Cl− concentration increased. On the other hand, the pH dependence of V′c100 showed a large change from pH = 2.0 to 2.5 when [Cl−] ≥ 0.5 mol·dm−3, but no pH dependence was observed in the pH ≤ 2.0 and pH ≥ 2.5 regions.
As for V′cMS, as shown in Fig. 7, it became less noble with increasing Cl− concentration as well as V′c100. On the other hand, the pH dependence of V′cMS was hardly observed, and it showed a constant value even when the pH was changed.
These results indicate that V′c100 and V′cMS are mainly depleted with increasing Cl− concentration. The pH dependence of V′cMS was not significant, while V′c100 changed rapidly in the pH range from 2.0 to 2.5.
3.2 Critical potential for crevice corrosion occurrence in artificial seawaterIn order to investigate the relationship between V′c100 and V′cMS calculated in the previous section and crevice corrosion occurrence, VCREV was investigated. The effect of electric potential on the current-time curves of SUS304 stainless steel specimens maintained at a constant potential in artificial seawater is shown in Fig. 8. In all tests, the current value decreased with the growth of the passive film, but at potentials above 184 mV, the current density increased due to crevice corrosion. On the other hand, the current density did not increase at 174 mV even though the potential was maintained for more than 14 days. Therefore, VCREV in this test was determined to be 184 mV.

The effect of holding potentials on current – time curves of SUS304 in an artificial seawater.
The effect of Cl− concentration in the bulk solution on VCREV is shown in Fig. 9, together with VCREV = 349 mV in 100 times diluted artificial seawater obtained in the same way. The results of VCREV in artificial seawater were almost identical to those of previously reported VCREV14) in NaCl solution. Each VCREV value was less than 400 mV, which is considerably less noble than V′c100 at pH ≥ 2.5 shown in Fig. 6, and close to V′cMS.

The effect of Cl− concentration on occurrence critical potential for crevice corrosion (VCREV) of SUS304 in aqueous chloride solutions.
Figure 10 shows the change with time of the spontaneous immersion potential of SUS304 stainless steel specimens immersed in various concentrations of aqueous CrCl3 solution degassed with Ar gas, and Fig. 11 shows the relationship between the spontaneous immersion potential and solution pH after 24 hours. At [CrCl3] ≧ 0.4 mol·dm−3 (pH ≦ 1.6), the spontaneous immersion potential remained less noble than the redox potential of hydrogen ions, indicating that the solution was in the active state. On the other hand, at [CrCl3] ≤ 0.2 mol·dm−3 (pH ≥ 2.1), the spontaneous immersion potential became nobler than the redox potential of hydrogen ions, indicating that the system was passive.

The change of free corrosion potential (Ecorr.) of SUS304 in CrCl3 solutions.

The relationship between solution pH and free corrosion potential (Ecorr.) of SUS304 in CrCl3 solutions.
In a degassed environment such as the one used in this measurement, the specimens are passivated when the reduction current of hydrogen ions exceeds the maximum value of the active dissolution current density (iCRIT), but it is difficult to completely remove oxygen from the aqueous solution by blowing in Ar gas, resulting in a reduction reaction of the small amount of dissolved oxygen remaining in the aqueous solution.15) Therefore, under passivation conditions, the spontaneous immersion potential becomes nobler than the redox potential of hydrogen ions.
3.4 Effect of Cl− and pH on current densityIn order to investigate how the solution inside the crevice affects the subsequent dissolution behavior of stainless steel and how the environment inside the crevice is changed, the active dissolution current density and the passive retention current density were summarized.
As in the case of V′c100 and V′cMS, iCRIT and passive retention current density (ipass) obtained from anodic polarization curves in NaCl + HCl aqueous solution are shown in Fig. 12. The iCRIT increased with increasing Cl− concentration, but the ipass did not. The pH dependence of both iCRIT and ipass was observed, and both increased with decreasing pH. ipass was independent of Cl− concentration and increased only with decreasing pH.

The effects of (a) chloride ion concentration and (b) pH on critical current density for passivation (iCRIT) and passive current density (ipass) of SUS304 in NaCl + HCl solutions.
V′cMS shown in Fig. 7 has no pH dependence and becomes less noble with increasing Cl− concentration. Similarly, VCREV is found to be desiccated by the increase in Cl− concentration outside the crevice as shown in Fig. 9. It was mentioned in section 3.1 that the values of V′cMS and VCREV were close to each other, but since Cl− was considered to be concentrated in the crevice at the time of crevice corrosion, the Cl− concentration of the solution in the crevice was estimated and then the two values were compared.
The Cl− concentration in the solution in the crevice was estimated and compared between the two values. From the current-time curve obtained in Fig. 8, the Cl− concentration up to the occurrence of crevice corrosion was calculated. Assuming that the amount of electricity until the onset of crevice corrosion is Q and the time is t, Q is calculated by eq. (4.1).16)
| \begin{equation} Q = \int_{0}^{t} i\cdot \text{d}t \end{equation} | (4.1) |
As a result of the calculation, Q ≈ 0.01 C was obtained regardless of the concentration of the artificial seawater and the holding potential. Since Cl− swims from the outside to the inside of the crevice to maintain electrical neutrality, the concentration of Cl− inside the crevice was calculated using eq. (4.2) for Q corresponding to the amount of dissolved metal ions. All swimming anions were considered to be Cl−, and the crevice volume V was calculated assuming a crevice thickness of 10 µm based on previous knowledge.17)
| \begin{equation} [\text{Cl$^{-}$}] = Q/96500/V \end{equation} | (4.2) |
As a result of the calculation, [Cl−] = 0.05 mol·dm−3 was obtained, and this concentration was added to the Cl− concentration before the test in the aqueous solution in the crevice. The concentration before the test was the same as that of the bulk aqueous solution. As a result, the Cl− concentration in the crevice was [Cl−] = (0.524 + 0.05) mol·dm−3 for artificial seawater and [Cl−] = (0.00524 + 0.05) mol·dm−3 for 1/100 artificial seawater.
The relationship between the Cl− concentration inside the crevice and VCREV obtained by the calculation is shown in Fig. 13, together with V′c100 and V′cMS for pH = 2.5 and pH = 2.0. It was found that VCREV and V′cMS have almost the same potential for all Cl− concentrations, but there is a large potential difference between V′cMS and V′c100 at pH = 2.5. It was also found that there is a large difference between V′c100 at pH = 2.0 and V′cMS at [Cl−] = 0.1 mol·dm−3, but V′c100 at pH = 2.0 are close to VCREV and V′cMS at [Cl−] ≥ 0.5 mol·dm−3.

The relationships between V′cMS, V′c100 and VCREV at estimated Cl− concentration in crevice solution.
Thus, the Cl− concentration dependence of VCREV and V′cMS, assuming the Cl− concentration in the solution in the crevice, is almost the same. There are some reports that the crevice corrosion initiation potential calculated by the dynamic potential method also agrees with the metastable pitting corrosion potential.18) These results suggest that micro pits observed at potentials above V′cMS can be the starting point of crevice corrosion in the crevice, while they can be repassivated to metastable pitting corrosion on the free surface.
4.2 Repassivation of metastable pitsAs shown in Fig. 13, where both V′c100 and V′cMS are shown, V′c100 became much less noble as the aqueous solution decreased to pH ≤ 2.0, and the difference between V′c100 and V′cMS became smaller. The pH = 2.0 is consistent with the general value5) of pH for deactivation of SUS304 stainless steel as mentioned in the introduction. In addition, as shown in Fig. 11, it was confirmed that SUS304 stainless steel was passivated in CrCl3 solution with pH ≥ 2.1. These results suggest that the decrease in V′c100 at pH ≤ 2.0 is related to the passivation ability of SUS304 stainless steel.
It has been confirmed that stable pits that grow stably on a free surface have a lid with an aperture of the same size as the micro pit and grow directly under the lid.9) It is considered that the starting point of both stable and metastable pits are micro pits, and those that are not repassivated, grow as stable pits, while those repassivated pits stop growing as micro pits and become metastable pits. In the environment of pH ≤ 2.0, the passivation ability of SUS304 stainless steel is low, so it is difficult to repassivate. Therefore, it is assumed that the generated micro pit easily grows as a stable corrosion pit, and V′c100 becomes less noble.
4.3 Role of micro pit as a starting point of crevice corrosionAs described in the previous sections, crevice corrosion of stainless steel is considered to be caused by the occurrence of micro pits, which can be repassivated on the free surface. The effects of micro pits on the free surface and crevice structure in neutral aqueous solution are summarized in Fig. 14.

The schematic diagram for effects of micro pits on free surfaces and crevice structures in neutral bulk solution.
When a micro pit occurs at the free surface, the solution inside the micro pit (metal chloride solution) diffuses into the bulk solution and dilutes the solution inside the micro pit, as shown in Fig. 14(A). Since the pH of the solution inside the micro pit immediately after the occurrence of the micro pit is much lower than 2.0,7,8) the size of the micro pit may grow to about 20–30 µm,9) and the surface around the micro pit may dissolve.7) Eventually, the aqueous solution around the micro pit is diluted to pH > 2.0 and is considered to be repassivated.
On the other hand, when a micro pit occurs inside the crevice, the solution inside the crevice diffuses only inside the crevice as shown in Fig. 14(B). Since the amount of the aqueous solution inside the crevice is minuscule and the pH is low due to passivation dissolution, it is not expected to increase by dilution. The solution in the crevice contains not only Cr3+ but also Fe2+ and Ni2+, and Cl− flows into the solution to maintain electrical equilibrium with these metal ions, so the Cl− concentration is higher than that of the CrCl3 solution used in this test, and the concentration of oxidant (dissolved oxygen) is considered to be more dilute than that of the aqueous solution degassed with Ar gas. Therefore, the pH at which SUS304 stainless steel is actually passivated is much higher than 2.0,12) and it is assumed that it is extremely difficult to passivate the micro pit again. In addition, metal chloride is concentrated by metal ions dissolved by the active dissolution of SUS304 stainless steel, and the inside of the crevice is filled with the solution inside the crevice with pH ≤ 2.0, and crevice corrosion is considered to progress.
When a micro pit is generated in the crevice, the extent of the metal chloride solution in the crevice is estimated. Assuming that the micro pit is a hemisphere of radius rm, the density of SUS304 stainless steel is ρ = 7.93 g·cm−3, and the molecular weight is M = 55.34 g·mol−1, the amount of metal dissolved in the micro pit is calculated by eq. (4.3).
| \begin{equation} m = 3/4\pi r^{3} \times 1/2 \times \rho/\text{M} \end{equation} | (4.3) |
If the saturated concentration of metal chlorides is C, the clearance interval is l, and the spreading radius of the metal chloride solution is R, then m is given by eq. (4.4) and R is obtained by eq. (4.5).
| \begin{equation} m = C \times l \times \pi \times R^{2} \end{equation} | (4.4) |
| \begin{equation} R = \{m/(C \times l \times \pi)\}^{1/2} \end{equation} | (4.5) |
If C = 4.3 mol·dm−3 (substituted by the saturated concentration of FeCl319)) and the clearance interval l is set to 10 µm as in Section 4.1, the radius of the micro pit is r ≈ 13–53 µm when r = 2–5 µm. In this study, the radius of the saturated metal chloride solution was calculated, but in reality, the concentration gradient exists, and it is assumed that a slightly wider range of metal dissolution is observed. It is thought that crevice corrosion progresses starting from the dissolution of metal in such a range.
4.4 Effect of Cl− and pH on crevice corrosionAs described in section 4.1, V′cMS and VCREV become less noble with increasing Cl− concentration. Therefore, the presence of Cl− facilitates the formation of micro pits and directly contributes to the crevice corrosion.
On the other hand, the decrease in pH prevents the repassivation of micro pits, but does not seem to be involved in the generation of micro pits, since it did not affect the value of V′cMS in Fig. 7. However, according to Fig. 12, ipass was not dependent on Cl− concentration, and increased with decreasing pH. The decrease of pH in the crevice of SUS304 stainless steel increases the passivation dissolution rate, which promotes electrophoresis of Cl− into the crevice and indirectly contributes to micro pit and crevice corrosion.
In the VCREV measurement, the surface of the specimen in the crevice was polished immediately before the test in order to facilitate the crevice corrosion generation. Therefore, the current until crevice corrosion occurs is measured as the dissolution current of the metal during the passivation process. Since we do not measure only the passive holding current density, we cannot simply discuss the passivation dissolution phenomenon. However, in a typical stainless steel crevice structure, there is no last minute polishing operation, and only the passivation-retained current density ought to be observed. Therefore, it is safe to conclude that the factor that increases the passivation dissolution rate in the crevice is the decrease in pH.
The pitting corrosion potential (V′c100) and metastable pitting corrosion potential (V′cMS) of SUS304 stainless steel in chloride solution were investigated, and compared with the critical potential for crevice corrosion initiation (VCREV) to study the role of micro pits as the starting point of crevice corrosion. In addition, the dependence of each potential and current value on Cl− concentration and pH was investigated to study the effect of these factors on the crevice corrosion initiation. A summary of the results is shown below.