2022 年 63 巻 3 号 p. 357-362
2022 年 63 巻 3 号 p. 357-362
Argon (Ar) plasma etching of stainless steel is known to form a unique surface texture consisting of nanopillars of several hundred nanometers due to the use of carbide precipitates as a template. The present study demonstrates that adding a small amount of nitrogen (N2) gas to Ar plasma discharge gas reduces the pillar size and enhances the pillar density, thereby resulting in a densely arranged nanoprotrusion surface. Admixing 1% N2 to the discharge gas decreased the height of each nanopillar to approximately a quarter of its original height. A further increase in N2 gas hardly changed the size of the nanoprotrusions, yet its number density increased up to 5% N2 addition. Admixed N2 gas generates N2+ species in the plasma, which form tiny chromium nitride (CrN) precipitates on a stainless steel surface. These CrN precipitates have become an alternative template for plasma etching. Nanoprotrusion surfaces are expected to improve the tribological properties of stainless steel surfaces; thus, the introduced process has potential for industrial applications.

Fig. 2 (a) Schematic illustration defining height, base diameter, cap diameter, and density of nanopillars and nanoprotrusion. (b) Height, base diameter, and cap diameter and (c) number density of the nanopillars across the different N2 gas concentrations.
Nanotextured surfaces of stainless steel have attracted attention from various industrial fields owing to their unique surface characteristics.1–4) For instance, textured surfaces consisting of nanopillars with sizes ranging from 200 to 1000 nm exhibit superhydrophilic, superhydrophobic, and antibacterial properties.5–7) Meanwhile, the surface with nanoprotrusions, which have a smaller size than nanopillars, improve tribological properties by reducing adhesion and friction.8–10) Furthermore, the characteristics are enhanced due to dence packing of nanopillars and nanoprotrusions on the surface.11–13) Therefore, surface engineering processes that can fabricate various nanotextured surfaces by controlling their size and density are required.
Argon (Ar) plasma etching is a process used to fabricate pillars with sizes ranging from nanometers to micrometers on various steel substrates.14–18) In this process, carbide precipitates, with a low etching rate, formed in the substrates during plasma etching act as templates for fabricating nanopillars. At the initial stage of etching, the substrate temperature is elevated by the bombardment of Ar+ included in the plasma. Subsequently, the formation of the precipitates is induced by accelerating the diffusion of solid soluble carbon (C) within grains and/or at the grain boundary, which causes a difference in the etching progress on the surface, resulting in the growth of nanopillars. According to this formation process, the size and number of precipitates are critical factors that affect the nanopillar size and density.17)
Such plasma etching can also form nanoprotrusions, which are basically nanopillars with smaller sizes, via carbide precipitates under mild etching conditions. Nakasa et al. revealed that the shape was converted from pillars to smaller protrusions on AISI 304 stainless steel by decreasing the power applied to generate Ar plasma using radio frequency (RF) discharge.19) We also previously reported that direct-current (DC) discharged Ar plasma etching with shortened process duration formed nanoprotrusions with a size below 200 nm on AISI 316 stainless steel (316SS) surfaces.18) However, the nanoprotrusions fabricated under such mild conditions are distributed sparsely on the surface. Such fabrication condition is beneficial for the formation of tiny precipitates due to a decrease in the substrate temperature elevation, though it prevents the dense arrangement of precipitates. Therefore, it is difficult to fabricate functional nanoprotrusion surfaces via carbide precipitates derived from solid soluble carbon contained in the steel substrates.
Based on the above considerations, supplying tiny precipitates that act as alternative templates during plasma etching and result in dense arrangement may be an effective method to solve this problem. To investigate such an inference, the present study focused on admixing nitrogen (N2) gas to Ar plasma discharge gas. N2 contained in the plasma facilitates the formation of Cr- and Fe-based nitrides, with a low etching rate similar to that of carbides, on the steel surface.20–25) Therefore, gas admixing likely supplies alternative templates, leading to the fabrication of nanoprotrusion surfaces.
In this study, we attempted to fabricate dense nanoprotrusion surfaces on stainless steel substrates via tiny nitride precipitates. For this purpose, N2 gas at small concentrations in the range of 1%–5% was admixed to Ar plasma discharge gas, and 316SS substrates, which are the study models, were plasma-etched. Thereafter, the variation in the surface characteristics with N2 admixing was evaluated in detail. The formation process of the nanotextured surface via plasma etching has been discussed with respect to the co-existence of N2 and Ar gas.
316SS (Nilaco Co., Japan) was selected as the substrate, and its chemical composition was 0.06 C, 0.70 Si, 0.98 Mn, 0.030 P, 0.005 S, 10.11 Ni, 16.75 Cr, and 2.05 Mo mass%, and Fe balance. The substrates were cut to samples of 10 × 10 × 1 mm3, and mirror-like polished using a colloidal silica suspension. Subsequently, the substrates were ultrasonically cleaned in ethanol for 10 min.
Plasma etching of steel substrates was carried out in a direct current (DC) glow plasma system built in our laboratory.26) Briefly, the cathode and anode electrodes were made of stainless steel and copper, respectively, which were set parallel at a distance of 20 mm in the vacuum chamber. The substrates were placed at the center of the cathode, and the pressure in the chamber was evacuated to less than 4.0 × 10−4 Pa. Gas mixtures comprising Ar and N2 were introduced into the chamber through mass-flow controllers up to a pressure of 300 Pa. The gas compositions were varied in the range of 0%–5% N2. Hereafter, the fabricated specimens are named according to the N2 concentration, for example, 3%N2. The gas was discharged using a DC power supply with a constant current of 0.10 A; subsequently, plasma etching was performed for 60 min. During the process, the applied voltage was varied accordingly within the range of 510–670 V. No temperature control of the substrates was conducted during the etching, but the temperature reached to the range of 783–823 K owing to energy transfer from the plasma. After the etching, the substrates were naturally cooled in a vacuum to ∼313 K to prevent rapid oxidation of the surfaces and were subsequently retrieved from the chamber.
2.2 Surface characterizationField-emission scanning electron microscopy (FESEM; JSM-6701F, JEOL, Japan) was used to observe the surface morphologies of the specimens. The acceleration voltage was 5 kV, and the specimens were placed at a tilt angle of 20°. The dimensions and number densities of the nanopillars were calculated from the acquired FESEM images using ImageJ software. The chemical states of the treated specimen surfaces were analyzed using X-ray photoelectron spectroscopy (XPS; PHI 5000 VersaProbe, ULVAC-PHI, Japan). Monochromatic Al Kα radiation (hν = 1486.8 eV) was used as the X-ray source, and the photoelectron take-off angle was set at 45°. The binding energies obtained from the spectra were corrected using the C 1s peak corresponding to naturally adsorbed hydrocarbons (284.8 eV). Spectra backgrounds were subtracted using Shirley’s method, and overlapping peaks were deconvoluted using a Gaussian–Lorentzian function. The optical emission spectra of the plasmas were measured using a spectrometer (USB2000+, Ocean Optics, USA). The crystal phase of the surface was determined by XRD using Bragg-Brentano geometry with Cu Kα radiation (XRD; New D8 ADVANCE, Bruker AXS, Germany).
Figure 1 shows the FESEM images of the 316SS surfaces plasma-etched using various gas compositions. The differences in the gas compositions significantly affected the dimensions and distribution of the fabricated nanotextured surface. Nanopillars were fabricated across the entire 0%N2 surface (Fig. 1(a) and 1(b)). Shorter pillars (indicated by the white arrows in Fig. 1(d)) were also formed across the 1%–5% N2 surfaces, and the shapes were similar among all these surfaces (Fig. 1(d), 1(f), and 1(h)). Meanwhile, mountain-like protuberances (indicated by the white dotted circle in Fig. 1(c)) were also observed on the 1%–5%N2 surfaces, which appeared to decrease in size with increasing N2 concentration.

FESEM images of plasma-etched 316SS surfaces: (a) and (b) 0%N2, (c) and (d) 1%N2, (e) and (f) 3%N2, and (g) and (h) 5%N2 surface.
The variations in the dimensions and number densities were evaluated quantitatively from the FESEM images using the dimensional parameters shown in Fig. 2(a). This evaluation allowed the determination of average height (nm), base diameter (nm), cap diameter (nm), and number density (µm−2) of the pillars; the corresponding values for each plasma-etched surface are presented in Fig. 2(b) and 2(c). The dimensions and number density were significantly influenced by the gas composition used for plasma generation. The pillar height, base diameter, and cap diameter were 618, 197, and 68 nm, respectively, on the 0%N2 surface. However, the height decreased considerably to 182 nm for the 1%N2 surface and continued to decrease gradually with increasing N2 concentration. The base and cap diameters of the 1%–5%N2 also decreased to approximately half of those measured on the 0%N2 surface, but there was no significant decrease with increasing N2 concentration among the 1%–5%N2 surfaces. The number density increased with increasing N2 concentration. Furthermore, the density on the 5%N2 surface was twice that on the 0%N2 surface. It can be concluded from these results that adding N2 gas decreased the dimensions of the pillar, and thus converted nanopillars to nanoprotrusions.

(a) Schematic illustration defining height, base diameter, cap diameter, and density of nanopillars and nanoprotrusion. (b) Height, base diameter, and cap diameter and (c) number density of the nanopillars across the different N2 gas concentrations.
The XPS narrow spectra corresponding to Fe 2p, Cr 2p, Mo 3d, and N 1s collected from the specimens are shown in Fig. 3. The Fe 2p3/2 spectra of all specimens were comprised an intense peak around 711 eV and a small shoulder peak around 707 eV, whereas satellite peaks derived from the Fe 2p3/2 level were not observed (Fig. 3(a)). A typical result of peak deconvolution analysis is shown in Fig. 3(e). The Fe 2p3/2 spectrum was deconvoluted into three peaks corresponding to metallic Fe (706.9 eV), Fe2O3 (710.5 eV), and FeOOH (711.9 eV).18,27,28) The binding energy of the main peak in the Cr 2p3/2 spectra was shifted toward the negative direction with the introduction of N2 gas, of which the width was broader than that of 0%N2 (Fig. 3(b)). The Cr 2p3/2 spectrum consisted of three peaks, corresponding to CrN (575.4 eV), Cr2O3 (576.4 eV), and Cr(OH)3 (577.9 eV).18,28,29) The chemical state of Cr was mainly oxide on the 0%N2 surface, whereas that of nitride and oxide on the 1%–5%N2 surfaces. The Mo 3d5/2 spectra were distinctly confirmed from the 1%–5%N2 surface, which were deconvoluted into three peaks attributed to Mo2N (229.0 eV), MoO2 (230.0 eV), and MoO3 (231.9 eV).30–32) The N 1s spectra obtained from the 1%–5%N2 surfaces overlapped with the Mo 3p3/2 spectra, which showed an intense peak around 397 eV, and the shape spread slightly to higher and lower energies (indicated by the black arrows in Fig. 3(d)). The three peaks corresponding to N 1s are contained in the overlapping spectra, which are in agreement with CrN (396.7 eV), Mo2N (397.8 eV), and NO (399.8 eV).26,30–33)

XPS spectra of (a) Fe 2p, (b) Cr 2p, (c) Mo 3d, and (d) N 1s and Mo 3p of plasma-etched surfaces and typical results of peak-deconvolution analysis (e), (f), (g), and (h).
The atomic ratios of CrN or Mo2N to Fe ([CrN]/[Fe] or [Mo2N]/[Fe]) on the plasma-etched surfaces calculated from the XPS spectra are shown in Fig. 4(a). The [CrN]/[Fe] ratio increased with increasing N2 concentration in the plasma. In contrast, the [Mo2N]/[Fe] ratio was close to zero, regardless of the N2 concentration. This suggests that the nitrides formed on the surface mainly consisted of CrN. Figure 4(b) presents the [CrN]/[Fe] ratios plotted against the number density of the nanopillars. A pronounced correlation was observed between the ratios and densities. Therefore, increasing the N2 concentration promoted the formation of CrN. Based on the results of FESEM and XPS analyses, it was revealed that nanoprotrusions mainly composed of CrN were fabricated on the 316SS surface via the plasma etching with admixing N2 gas at small concentration.
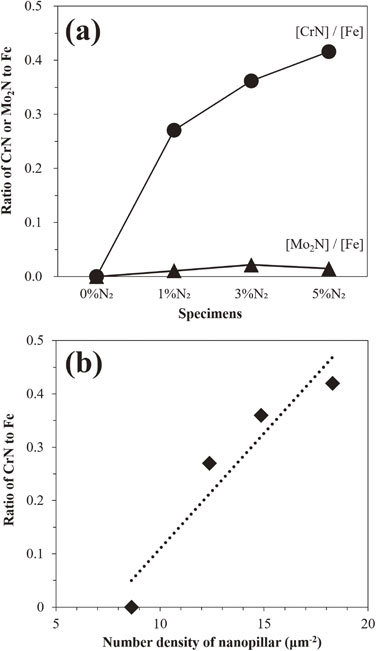
(a) The atomic ratio of CrN or Mo2N to Fe calculated by XPS spectra for the plasma-etched surface. (b) Correlation between the ratio of CrN and Fe and number density of nanopillars and nanoprotrusion surfaces.
To further understand the effect of admixing N2 gas on the formation process of nanoprotrusions, optical emission spectra and XRD measurements were performed. Typical optical emission spectra obtained by the plasmas corresponding to 0%N2 and 3%N2 are presented in Fig. 5(a). The major features corresponding to the Ar line were located in the wavelength range of 690–850 nm for the 0%N2 plasma, and the peak at 488 nm, observed in the weak band between 400 and 500 nm, was attributed to Ar+, which is a key species for plasma etching.34–36) In addition to the Ar band, intense features of the N2 line appeared for the 3%N2 plasma, including peaks attributed to N2+ (391.4 nm) that contribute to plasma nitriding.37,38) The peak intensities of N2+ increased with increasing N2 concentration, whereas there was no notable difference in Ar+ intensities among the different plasmas (Fig. 5(b)).

Optical emission spectra of plasmas corresponding to 0%N2 and 3%N2. (a) Full spectra in the range of 200–900 nm and (b) peak intensities of optical emission spectra at N2+ and Ar+ plotted against the specimens.
Figure 6 shows the XRD patterns of 0%N2 and 3%N2 surfaces as they depict typical features of plasma-etched surfaces. On the 0%N2 surface, the peaks corresponding to γ-Fe and M23C6 carbide phases were observed.39,40) The peak width corresponding to γ-Fe was broadened as compared to that of untreated surface due to Ar plasma etching. Ar+ bombardment leads to the formation of many point defects such as vacancies in the steel matrix,41) which are responsible for the change in the peak width. The peaks corresponding to γ-Fe, α-Fe, and CrN were evident for the 3%N2 surface,39) whereas that of the carbides disappeared. The width of the γ-Fe peak was broader than that of the peak in 0%N2 surface, attributing to lattice expansion with the incorporation of interstitial N atoms, besides defect formation.39,40,42) Furthermore, the appearance of α-Fe peak supported phase transformation of γ-Fe expanded by N atoms via the formation of CrN precipitates during the plasma etching.41–43) Correlative variations in the XRD patterns indicated that the formation of CrN instead of carbides was facilitated by admixing N2 gas.

XRD patterns of 0%N2, 3%N2 and untreated surface.
These analytical results suggest that admixing a small amount of N2 to Ar plasma discharge gas resulted in the fabrication of nanoprotrusion surface on the 316SS substrates through the process described as follows. At the initial stage, the bombardment of Ar+ in the plasma led to the formation of point defects and elevated the substrate temperature above 773 K (Fig. 6). Simultaneously, N2+ was dissociated to N atoms through collisions on the surface, after which they penetrated the substrates. The diffusion of Cr atoms in the matrix and the penetration of N atoms was accelerated due to the defects and higher temperature, leading to the development of CrN precipitates with a lower etching rate than that of the matrix. The precipitates functioned as a template to prevent etching, and thus, the matrix around the precipitates was preferentially etched. However, the bare surface formed by the etching was nitrided immediately by N2+ present in the plasma, thereby preventing growth in the vertical direction. Consequently, nanoprotrusions composed of CrN were formed on the 1%–5%N2 surfaces.
Furthermore, the intensity of N2+ in the plasma was correlated with the number density of the nanoprotrusions (Figs. 2(c) and 5(b)). The results suggest that the nitriding reaction was facilitated by increasing N2+ in the plasma, resulting in an increase in the formation of CrN precipitates on the 316SS surface. The amount of precipitates is a crucial factor affecting the number density on the resultant surface;17) therefore, the concentration of N2 gas introduced in Ar gas is a principal parameter for controlling the density of the nanoprotrusion surface.
It was demonstrated that a nanoprotrusion surface fabricated by plasma etching and admixing N2 gas at a small concentration to Ar plasma discharge gas should provide several advantages compared to the conventional etching process using pure Ar gas. In particular, the proposed process can fabricate nanoprotrusions with a size of approximately 200 nm, which contribute to the reduction of surface friction.10,44,45) Furthermore, the nanoprotrusions are composed of CrN, which is a hard material, thereby improving the wear resistance of the 316SS surface.46,47) We further plan to evaluate the tribological properties of the nanoprotrusion surface to demonstrate these advantages; the results of the evaluations will be reported in the near future.
This is the first study that reports the formation of nanoprotrusion surfaces via nitride precipitates as templates using plasma etching. The study revealed that admixing a small amount of N2 to Ar plasma discharge gas induced the formation of nanoprotrusions composed of CrN. The concentration of N2 was the principal parameter affecting the dimensions and number density of the resultant surfaces. In fact, the dimensions, including the height, base diameter, and cap diameter, decreased considerably with the addition of 1% N2 gas; in contrast, the density increased with an increase in the concentration. This is because the N2+ species contained in the plasma facilitated the formation of tiny CrN precipitates. In conclusion, these findings demonstrate that the proposed process can result in the fabrication of a variety of nanotextured surfaces by Ar plasma etching, which is expected to improve the tribological properties of stainless steels.
The authors gratefully acknowledge Mr. Yamane and Mr. Tokuda from the Kitami Institute of Technology for their help with the XPS analysis and FESEM observations, respectively.