2022 年 63 巻 3 号 p. 389-393
2022 年 63 巻 3 号 p. 389-393
Ultra-lightweight ceramics were fabricated by firing the mixtures of pork bone (PB) and incinerated ash of sewage sludge. PB content in the mixtures was varied from 0 to 15 mass%. The mixtures were pressed in a discus body (4 cm in diameter) and the body was fired at the temperature range from 1000 to 1150°C for 1 h in air. Bloating behaviors were observed in the samples fired at the temperature over 1050°C. The remarkable lightweight ceramics, which have the apparent porosity less than 1.0 g cm−3, were obtained by the addition of 10–15 mass% PB and the firing at the temperature over 1050°C. Diffuse reflectance spectra revealed that ferric oxide in the samples fired at the temperature over 1050°C was reduced to ferrous oxide. It was considered that the large bloating in the PB added samples occurred due to both the evolution of oxygen from ferric oxide and the oxidation of residual carbon derived from PB inside the samples after melting of the surface at high temperatures.

Generating amount of sewage sludge (SS) is increasing year by year according to the propagation of public sewerage and SS is incinerated or melted to reduce the volume. Therefore, sewage sludge ash (SSA) is also increasing year by year. Although SSA and dried SS are often landfilled, places capable of being used in landfill are limited. SSA is often used as raw material for production of cement. In addition, recycled bricks made from SSA have been produced in several municipalities so far. However, continuous management for recycled bricks is very difficult because the production of it takes a high cost compared with commercially available bricks. Now, development of effectively using methods of SS and SSA is required from local municipalities all over Japan.
Because such a situation is the same for other countries, many studies about the recycling of SS and SSA have been reported in each country so far. Several studies have reported on mixtures of SS and clay, such as, the production of lightweight ceramic bricks,1) the mechanism of bloating of ultra-lightweight ceramics,2) and the effect of forming SS on the properties of lightweight ceramics.3) In addition, the studies about lightweight bricks fabricated from SS and rice husks4) and the high-porosity ceramics for SS using starch on CaCO35) as forming agent were reported. The works about recycled ceramics using only SSA,6) such as, the microstructure and leaching of sintered SSA and the effect of heating temperature on the sintering characteristics of SSA were reported.7,8) Furthermore, many studies have been reported on the SSA with additives. Park made glass ceramics using fly ash from SSA.9) Tsai investigated the characteristics of lightweight aggregates sintered from SSA added with SiO2–Al2O3 flux.10) Chiou fabricated lightweight aggregates from SS and SSA.11) Merino studied the ceramic characteristics of SSA mixed with various additives.12) Lin investigated the effect of sintering temperature on water retention characteristics of SSA - diatomite based porous ceramics.13)
The authors fabricated foamed porous ceramics from SSA mixed with fly ash which was discharged from a thermal power plant.14,15) The ceramics were fired in nitrogen atmosphere due to make porous ceramics. The authors considered that carbon derived from remained at high temperature and acted as a foaming agent. For practical use for recycled SSA, it is desirable to fabricate porous ceramics by firing in air because firing in nitrogen atmosphere takes a high cost of the special equipment of an existing furnace.
Therefore, the authors focused on use of dried pork bone (PB) as the other foaming agent. The powder obtained by burning the bone of an animal has been commonly used as a fertilizer and raw material of pottery so far because the powder contains phosphorus and calcium. Now, more than 450 tons of PB is wasted every year in Fukuoka prefecture, Japan. The most part of wasted PB is disposed after incineration except for use in a fertilizer. The unbaked PB is expected to act as a foaming agent in case of firing in air because the PB contains a large amount of organic matter and the carbide derived from organic matter will be remained up to high temperature.
The addition of PB is advantageous to use for roof greening materials because PB abundantly contains calcium and phosphor which are useful for a plant growth. The present paper attempted to fabricate the foamed porous ceramics form mixtures of PB and SSA by firing in air in order to turn into commercial application.
SSA which was provided from Kyoto City Waterworks Bureau was used as a raw material. The chemical composition of SSA is shown in Table 1. SSA consists mainly of about 35 mass% of SiO2, 27 mass% of Al2O3, 17 mass% of P2O5, 9 mass% of Fe2O3 and 5 mass% of CaO. P2O5 and CaO are derived from an organism in sewage. Fe2O3 is acted as a colorant of samples. PB was provided from the Fukuoka Research Commercialization Center for Recycling Systems. The chemical composition of inorganic component of PB is shown in Table 1. PB consists mainly of about 60 mass% of CaO and 37 mass% of P2O5. Thermal gravimetric (TG) and differential thermal analysis (DTA) for PB powder is shown in Fig. 1. The elimination of hydration (about 100°C) and the combustion of organic substance (250–600°C) were observed in the TG and DTA curves as shown in Fig. 1. DTA-TG analysis resulted that 35 mass% of organic substance and 5 mass% of water were included in PB and organic substance was burnt down under 600°C in case of PB alone. However, in case of mixture of SSA and PB, it is assumed that a small amount of carbide remains up to high temperature because the sintering of SSA could suppress diffusion on oxygen which oxidizes an organic substance.

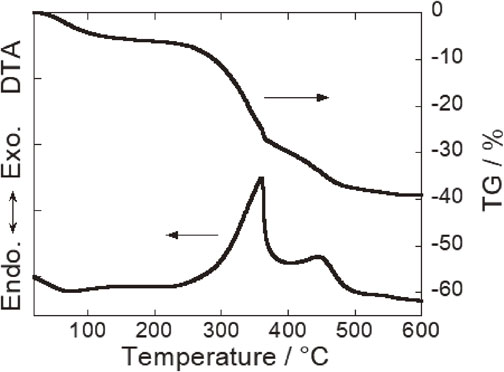
DTA-TG curves of pork bone.
SSA and PB were crushed under 75 µm and thoroughly mixed in a mortar with water. Amount of addition of PB to SSA was varied from 0 to 15 mass%. Hereafter, samples are called PB-x according to addition amount (x) of PB. For example, PB-15 stands for the sample added with 15 mass% PB. Dried mixture was pressed into disks of 4 cm in diameter under the pressure of 10 MPa. The disks were placed in a tubular electric kiln and fired at the temperature range of 1000 to 1150°C for 1 hour in air.
Thermal gravimetric (TG) and differential thermal analysis (DTA) for PB was performed using TG-DTA analyzer (Rigaku TG8120). The bulk volume was estimated by measuring the diameter and thickness of fired samples. The bulk and apparent density, and apparent porosity of fired samples was measured by the Archimedes method. The true density of the samples was measured by using a 25 ml glass specific gravity bottle. Phase identification was carried out by X-ray diffraction (XRD) using MiniFlex 600 (Rigaku, Japan). The microstructure of the sintered samples was observed using scanning electron microscopy (SEM, JEOL JSM-7600). Diffuse reflectance spectra in the wavelength range of 400 to 1500 nm using Al2O3 as a reference were measured using spectrophotometer (Shimadzu, UV-2100).16)
Figure 2 shows XRD patterns of the samples fired at 1125°C. As shown in Fig. 2 peaks of α-quartz, α-cristobalite, hematite, mullite and other crystalline phases were observed in all samples. The existence of amorphous phases in all samples was assumed due to the rise of background in 20–30 two theta region. The intensity of the peaks of the crystalline phases decreased in an increase of the addition amount of PB. This result was indicated that the addition of PB accelerated the melt at the temperature over 1100°C.

XRD patterns of the samples fired at 1125°C. Each mark, ○: α-quartz, ●: hematite, □: α-cristobalite, ■: albite, △: mullite, ▲: anorthite, respectively.
Figure 3 shows the relationship between the bulk volume and firing temperature. The bulk volume decreased at the temperature range from 1000 to 1050°C and then increased with an increase in temperature above 1050°C without depending on the addition amount of PB. An increase rate of bulk volume enlarged accompanied with the addition amount of PB. The decrease in bulk volume at lower temperature occurred due to sintering of samples. On the other hand, the increase in bulk volume at higher temperature suggested that the sample expanded due to the gas evolution inside of samples because bulk volume varied depending on the firing temperature and the addition amount of PB. It is assumed that the gas evolution due to remained carbide derived from PB was occurred because the rate of an increase of it for the PB added samples was larger than that for the samples without PB. Especially, the bulk volume of PB-15 fired at 1150°C was four times as large as PB-15 fired at 1050°C. In addition, the maximum bulk volume was approximately three and a half as large as that of unfired sample PB-15 (2.8 cm3).

Relationship between the bulk volume and firing temperature.
In the studies about the bloating of SSA, it was reported that the reduction of Fe3+ to Fe2+ involved that the release of oxygen gas at high temperature.6,7,19) Then, it was assumed that the samples without the addition of PB expanded due to the oxygen gas evolution which was caused by the decomposition of Fe2O3 in SSA.
Figure 4 shows the SEM images of a cross section of the samples fired at the temperature range from 1100 to 1150°C. Large closed pores ware observed inside the PB added samples. In addition, the part of surface, which was smooth due to be melt, for PB-10 fired at 1100°C was observed and the smooth surface seems to be melted. Furthermore, the inside pf PB added samples seems to be melt from observation of the shape of the close pores. The development of pores of PB added samples, accompanied with an increase in temperature and the addition amount of PB, was observed. The expansion shown in Fig. 3 assume to be due to this development of pores inside samples.

SEM images of the cross section of the samples fired at (a) 1100, (b) 1125 and (c) 1150°C.
Figure 5 shows the relationship between the bulk density and firing temperature. The bulk density increased at the firing temperature range from 1000 to 1050°C and then decreased above 1050°C without depending on the addition amount of PB. This result is inclined to the opposite to the result of bulk volume because bulk density is in inverse proportion to bulk volume when the weight of the sample is constant.

Relationship between the bulk density and firing temperature.
Figure 6 shows the relationship between the apparent density and the firing temperature. The apparent density of PB-0 and PB-3 similarly varied as an increase in temperature. On the other hand, the apparent density of PB-10 and PB-15 significantly decreased at the firing temperature above 1050°C and reached to 1.0 g cm−3. Therefore, ultra-lightweight ceramics which were able to float on water was fabricated successfully. The decrease of apparent density suggests an increase in the volume of closed pore inside of samples. These results indicated that carbide derived from PB was remained up to 1100°C and oxidation of carbide caused gas evolution inside of samples.

Relationship between the apparent density and firing temperature.
However, the true density of the fired samples was almost constant regardless of addition of PB and it was around 2.63 g cm−3.
3.4 Apparent porosityFigure 7 shows the relationship between the apparent porosity and firing temperature. The apparent porosity of PB-0 and PB-3 similarly varied as an increase in temperature. On the other hand, the apparent porosity of PB-10 and PB-15 decreased significantly at the firing temperature range from 1000 to 1100°C and then increased with an increase in temperature above 1100°C. The apparent porosity of PB-10 and PB-15 reached almost 0% at the firing temperature of 1100°C. The results of PB-10 and PB-15 indicated that open pore disappeared due to melting the surface of samples as mentioned from SEM images in Fig. 4. This result was associated with the results of significant decrease of apparent density of PB-10 and PB-15 at the same firing temperature. However, open pores formed at the firing temperature over 1100°C. It was suggested that generated gas in the inside of samples broke through the melt surface layer of samples.

Relationship between the apparent porosity and firing temperature.
The color of the samples became red at the firing temperature range from 1000 to 1050°C and then changed into brown over 1050°C without depending on the addition amount of PB. The color variation of the samples is assumed to be due to the variation of chemical states of iron ions in the samples. Then, diffuse reflectance spectra of the samples were measured to clear the variation of chemical states of iron ions. Figure 8(a)–(d) shows the diffuse reflectance spectra (DRS) of powders which were obtained by pulverizing fired samples. F(R) value is equivalent to intensity of absorption and the intensity was very strong in the wavelength range from 400 to 600 nm without depending on the addition amount of PB. This absorption peak is mainly attributed to Fe3+ ion.17) The F(R) values in the range over 800 nm tended to increase as an increase in firing temperature. It was reported that the absorption attributed to Fe2+ revealed around 1200 nm.18) Then, the relationship between F(R) value at 1200 nm (denoted by F(R)1200) and firing temperature is shown in Fig. 9.

Diffuse reflectance spectra of (a) PB-0, (b) PB-3, (c) PB-10, (d) PB-15.
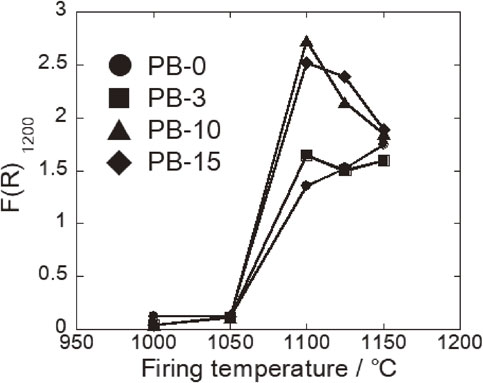
Relationship between the F(R) value at 1200 nm and firing temperature.
The value of F(R)1200 was almost zero at the firing temperature range from 1000 to 1050°C without depending on the addition amount of PB. On the other hand, the value was very large at the temperature above 1050°C. In addition, the values of PB-10 and PB-15 were larger than those of PB-0 and PB-3 at the firing temperature over 1050°C. In case of PB-0, it is considered that the formation of Fe2+ was caused by the decomposition of Fe2O3. Therefore, the value of F(R)1200 of the samples with the addition of PB was added the value due to the Fe2+ formed by the decomposition of Fe2O3 because the decomposition is assumed to be occurred in the samples with the addition of PB. It is considered that Fe3+ was reduced to Fe2+ by residual carbon derived from PB at the temperature over 1100°C. However, the value of PB-10 and PB-15 decreased at the temperature over 1100°C. It is considered that a part of Fe2+ ions was oxidized to Fe3+ ions because of the formation of open pores at the surface of the samples as shown in Fig. 7.
3.6 Mechanism for bloating of SSA added with PBPrevious studies have been reported about the bloating mechanism of sintered SSA so far. Cheesman considered that the formation of a bubbled microstructure was due to softening of the glassy phase together with evolution of gasses generated by the decomposition of inorganic phases.6) Tsai reported that the surface of SSA started to melt in the glass phase and bloat.10) Lin suggested that a result of the softening of the glassy phase in the system, along with a concurrent evolution of gas at high temperature.7) The reduction of Fe3+ to Fe2+ involves the release of oxygen. In addition, studies on the bloating mechanism of mixture of SS and SSA were reported by Chou et al. They assumed that SS supplied more carbide, which helped to process an oxidation reaction reached with ferric oxide, and generated CO2 and CO to enhance the expansion.11) Chiang manufactured the lightweight bricks from SS and rice husk and reported that the larger the rice husk organic matter content, the greater the porosity.4) At the temperature of 1100°C open porosity of the specimen increased from 5% to 38% with an increase in added rice husk from 0 to 20%. The result indicated that organic compound derived from rice husk remained up to 1100°C.
The bloating mechanism in the present study was considered based on the previous studies above mentioned. We focused on the bloating behavior of the samples fired at 1100°C because the result of the bulk volume and bulk density clarified that the change from contraction to expansion occurred at the firing temperature range from 1050 to 1100°C without depending on the addition amount of PB. At first, the results shown in Fig. 3, Fig. 4 and Fig. 6 indicated that the bloating of the samples without PB occurred at the firing temperature range from 1050 to 1100°C. It was considered that the glassy phase formed inside the samples and formed by oxygen gas generation from the decomposition of Fe2O3.6,7,19) This mechanism was supported by the result of DRS as shown in Fig. 9.
Next, the mechanism of a large expansion for samples PB-10 and PB-15 was considered as follows. Almost whole amount of organic compounds contained in PB-10 and PB-15, was oxidized during heating up to 1050°C. However, only minimal part of organic compounds could remain as a carbide ever over 1050°C because the oxidation of organic compounds could be protected due to suppress oxygen diffusion during sintering mixtures of SSA and PB. As with the samples without PB, oxygen gas evolved due to decomposition of Fe2O3 inside the melted samples added with PB at firing temperature of 1100°C. The carbide which remained up to 1100°C was oxidized by the oxygen gas and then evolved CO and CO2 gas caused to bloat PB added samples. As shown in Fig. 9, Fe2+ in samples PB-10 and PB-15 existed more than that in samples without PB and the results indicated that reduction of Fe3+ to Fe2+ by CO gas from inside the samples PB-10 and PB-15.
Mixtures of SSA and PB were fired at the temperature range from 1000 to 1150°C in air. The expanding behaviors for fired samples were investigated by measuring bulk volume, bulk density, apparent density, apparent porosity, and diffuse reflectance spectra. The following results were obtained.