2022 Volume 63 Issue 4 Pages 458-461
2022 Volume 63 Issue 4 Pages 458-461
To suppress the over-oxidation of solvents, reaction conditions were optimized, and ligands were added to the system to facilitate the oxidation of sulfur compounds with molecular oxygen. The addition of suitable nitrogen ligands led to effective suppression, and 2,2′-bipyridine showed the best performance among the analyzed samples. After the use of the ligand, the yields of by-products from solvent oxidation were reduced by a factor of 15 compared to those from the reaction without any ligands.
To suppress the over-oxidation of solvents, reaction conditions were optimized, and ligands were added to the system to facilitate the oxidation of sulfur compounds with molecular oxygen. The addition of suitable nitrogen ligands led to effective suppression, and 2,2′-bipyridine showed the best performance among the analyzed samples. After the use of the ligand, the yields of by-products from solvent oxidation were reduced by a factor of 15 compared to those from the reaction without any ligands.
Owing to an increase in environmental concerns, considerable efforts have been devoted to the reduction of the content of organosulfur compounds in transportation fuels because these compounds produce sulfur oxides (SOx) during their combustion. Currently, researchers are investigating the development of highly active catalysts for the ultra-deep hydrodesulfurization (HDS) of diesel fuels to meet the sulfur regulation. Oxidative desulfurization (ODS) has been proposed as an alternative method for deep desulfurization. ODS was first proposed in 1990s.1–3) In the ODS process, the oxidation of sulfur compounds is a key step, and many studies concerning the oxidation of sulfides have been published. Usually, peroxides (peracids, organic peroxide, or hydrogen peroxide) are used for the oxidation of organic sulfides to sulfones because the reactions proceed rapidly and selectively.3–9) However, the use of peroxides is hazardous and therefore is not suitable for large-scale applications and storage. Therefore, the development of a new oxidation system without peroxides is required.
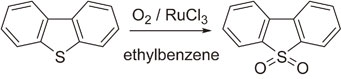
In a previous study,10,11) dibenzothiophene (DBT) and related compounds were oxidized to the corresponding sulfones using molecular oxygen in the presence of a ruthenium catalyst in hydrocarbon solvents under mild conditions (eq. (1)).
 | (1) |
 | (2) |
 | (3) |
 | (4) |
Gas chromatography (GC) and gas chromatography-mass spectrometry (GC-MS) analyses were conducted on a Shimadzu GC-2025 (with a CBP-1 column and a frame ionization detector) and Shimadzu GCMS-QP2010 instrument, respectively. All reagents employed in this study were commercially available and were used without further purification.
2.2 OxidationThe typical procedure for oxidation are as follows: a mixture of DBT (184 mg, 1 mmol) and ruthenium chloride (10 mg, 0.05 mmol) in EB (20 g) was stirred in an oil bath (at 80°C) for 20 h under a static pressure of oxygen (1 atm). After the completion of the reaction, the product mixture was analyzed using GC and GC-MS.
First, the effects of reaction temperature were examined. A mixture of DBT and EB was stirred at 60–90°C under a static pressure of oxygen (1 atm) for 20 h. After the reaction, the product mixture was analyzed using GC and GC-MS. The only product obtained from DBT was dibenzothiophene-5,5-dioxide (sulfone). As a result of the oxidation of the solvent, a mixture of 1-phenylethyl hydroperoxide (A), 1-phenylethanol (B), and acetophenone (C) was observed. These results are consistent with the reaction mechanism shown in eqs. (2)–(4). Among the products from solvent oxidation, the yield of (C) was higher than those of (A) and (B); approximately 99% of the products were (C).
 |
| \begin{align} &\textit{conversion of DBT ($\boldsymbol{X}$)} \\ &\quad = \left(1 - \frac{\textit{DBT remained}\ (\textit{mol})}{\textit{DBT employed}\ (\textit{mol})}\right) \times 100 \end{align} | (5) |
| \begin{align} & \textit{yields of the solvent} - \textit{oxidized products ($\boldsymbol{Y}$)} \\ &\quad =\left(\frac{\textit{summation yields of the products $\boldsymbol{A},\boldsymbol{B},\boldsymbol{C}$}\ (\textit{mol})}{\textit{solvent employed}\ (\textit{mol})}\right) \\ &\qquad\times 100 \end{align} | (6) |

Temperature dependence on the conversion of DBT (circle) and the yields of the products from the solvent-oxidation (square). [RuCl3]:[DBT] = 0.05:0.25 (in mmol), EB 20 g, 70 hr, under static pressure of oxygen.
To control the reaction, the effects of the ligands were examined. The addition of appropriate ligands to the system is known to change the product distribution. The authors conducted the oxidation of DBT in the presence of typical N-, O-, and P-ligands. The results are presented in Table 1. The addition of ligands led to a lower X value than those observed in the reaction without any ligands. In these cases, the reaction was conducted at an increasing temperature to obtain an efficient DBT conversion. Neither oxygen nor phosphorus ligands could suppress the over-oxidation of the solvent. Among the nitrogen-containing ligands examined, monodentates, such as pyridine and its derivatives, showed negligible or no effect on the suppression of the over-oxidation of the solvent, while bidentate compounds, such as 2,2′-bipyridine (bpy) and 1,10-phenanthroline (phen), effectively suppressed the over-oxidation of the solvent.

To obtain precise information concerning these bidentates, the auto-oxidation of EB was conducted with molecular oxygen in the presence of ruthenium chloride at 90°C. The results are shown in Fig. 2. Without bidentate ligands, EB was oxidized to (C) in 12 mol% yield within 6 h. However, its yield reduced to 1.8–3.5 mol% at 6 h in the presence of bpy or phen. These results indicate that the addition of a suitable quantity of nitrogen-bidentate ligands suppresses the over-oxidation of the solvents.
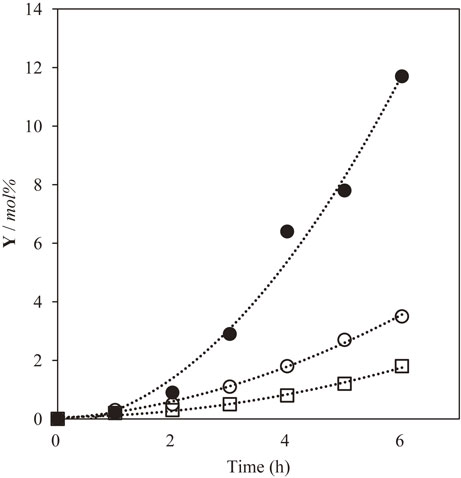
Time course of the oxidation of ethylbenzene in the presence of ruthenium chloride and bpy or phen under oxygen. [RuCl3]:[additive] = 0.05:0.0125 in mmol, [EB] = 20 g, 90°C, [additives] = none (filled circle), bpy (open circle), and phene (open square).
To obtain precise information, the reaction was conducted using different concentrations of ligands. The results are shown in Fig. 3. In the case where phen was used as the ligand, the reaction temperature was set at 90°C to obtain efficient DBT conversion. The addition of a very small quantity of ligands (0.0125 mmol) to the system led to a substantial decrease in the yields of the products from solvent oxidation. The yield reductions ranged from 18% to 2% and from 24% to 5% with bpy and phen, respectively. DBT conversion was maximum in the reactions with moderate quantities of ligands (0.02–0.03 mmol). This quantity of ligands (0.02–0.03 mmol) is approximately half the quantity of the catalyst (0.05 mmol). The best results, DBT conv. = 99% and solvent-oxidation = 1.2%, were obtained in the reaction with 0.03 mmol of bpy.
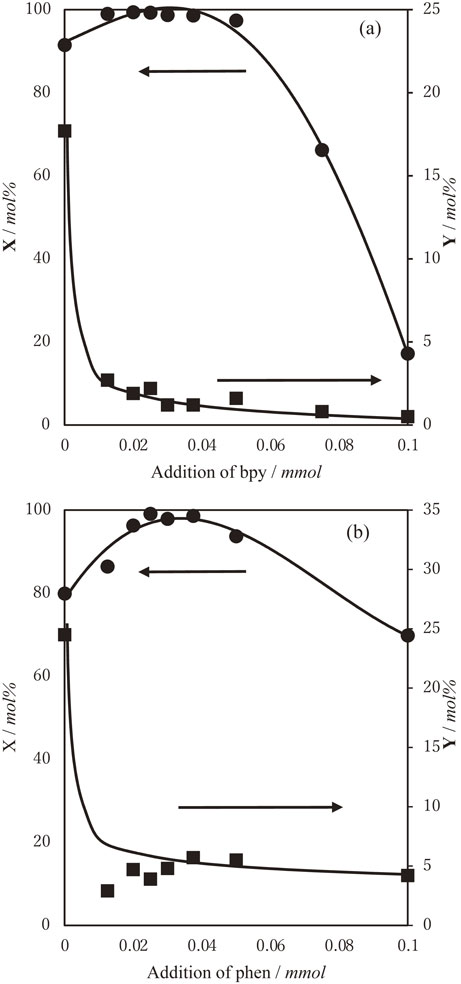
Oxidation of DBT with molecular oxygen in the presence of ruthenium chloride and bpy (a) or phen (b) in ethylbenzene. [RuCl3]:[DBT] = 0.05:0.25, EB 20 g, 70°C (for bpy) or 90°C (for phen), 20 h, under static pressure of oxygen. Conversion of DBT (circle), yields of the products from solvent oxidation (square).
In the reaction system, the ruthenium catalyst might be in equilibrium between the dissociation and coordination states of the ligand (eq. (7)).
 | (7) |
The authors investigated the oxidation of DBT with molecular oxygen in the presence of a RuCl3 catalyst and nitrogen ligands, and the following conclusions were drawn.
This study was supported by JSPS KAKENHI (Grant Number 19K05563).