2023 Volume 64 Issue 10 Pages 2394-2399
2023 Volume 64 Issue 10 Pages 2394-2399
Zinc-Air Batteries (ZABs) are a promising solution for grid-scale storage. In this work, ZAB chemistry is reviewed and the role of catalysts at the cathode is explained. Transition metal oxides are an economical substitute for noble-metal based catalysts. However, their poor electrical conductivity requires additional efforts in catalyst activation. This is commonly done by reducing particle size and increasing electrical access which can be done simultaneously by hybridizing with carbon derivatives such as reduced graphene oxide.
As the world weans off its dependence on fossil fuels, grid-scale energy storage becomes increasingly important to efficiently utilize intermittent renewable energy sources. In the absence of any energy storage solution, excess renewable energy capacity is simply curtailed. Using zinc, a common element in Earth’s crust, as the anode, rechargeable zinc-air batteries (ZABs) can be manufactured on a large scale with a low cost.1) The theoretical gravimetric energy densities of various rechargeable batteries are summarized in Fig. 1, in which metal-air batteries such as ZABs outperform conventional lead acid and lithium-ion batteries.2) The aqueous electrolyte also makes them resistant to fires which is beneficial as renewable energy generation sites such as solar farms and wind mills are often located far away from population centers.

Theoretical energy density of different batteries and gasoline. (Reproduced from Ref. 2).)
A schematic illustration of a ZAB is shown in Fig. 2. Crucial in metal-air batteries is the Gas Diffusion Layer (GDL). The GDL allows oxygen in the air to diffuse through its porous structure to be reduced at the cathode, which is commonly referred to as the oxygen reduction reaction (ORR). During the charging process, oxygen evolution reaction (OER) occurs.

Schematic principle of operation for ZABs. (Reproduced with permission from Ref. 56). Copyright 2022 The Royal Society of Chemistry)
During discharging, ORR occurs at the cathode of a ZAB. This reaction can occur via either a 2-electron mechanism or a 4-electron mechanism. In basic electrolyte,3)
| \begin{equation} \text{O$_{2}$} + \text{2H$_{2}$O} + \text{4e$^{-}$} \Rightarrow \text{4OH$^{-}$}\quad E^{0} = 1.23\,\text{V}\ \text{vs.}\ \mathrm{RHE} \end{equation} | (1) |
| \begin{align} \text{O$_{2}$} + \text{H$_{2}$O} + \text{2e$^{-}$} &\Rightarrow \text{HO$_{2}{}^{-}$} + \text{OH$^{-}$}\\ &\qquad \qquad \quad E^{0} = 0.76\,\text{V}\ \text{vs.}\ \mathrm{RHE} \end{align} | (2) |
In order to achieve higher efficiency, the 4-electron mechanism (eq. (1)) is preferred due to a lower overpotential. By catalyzing and improving the selectivity of the 4-electron pathway over the 2-electron pathway (eq. (2)), it is possible to unlock the potential of ZAB chemistry for greater efficiency.
Rechargeable ZAB provides a low cost and environmentally friendly solution for grid-scale storage.4) However, despite its high specific energy density, ZABs are limited by its low current density due to the slow kinetics of the ORR5) and high overpotentials of the OER6) during the discharge/charge process respectively. The increase in ORR overpotential is even more evident at high discharge current densities above 30 mA cm−2.7) This is addressed commercially with the use of well-established noble metal catalysts such as Pt for ORR8) and RuO2 for OER.9) However, these involve the use of scarce and expensive noble metals. Since catalytic activity is limited to the surface of the catalyst, utilization of such noble metal catalysts is maximized by increasing their specific surface area.10) This has been achieved by synthesizing nanoparticles11) or depositing single atoms on an electrically conductive substrate.12)
Various d-transition metal oxides and their hydroxides with promising electrocatalytic performances have been reported and they offer possible substitutes for the catalysts of precious metals.13–16) The catalysts of these transition metals can be formulated in bimetallic compositions to utilize the unique electronic configuration of transition metals,17–21) resulting in catalytic performance superior to the single metal catalysts.18) One such example is evident in the results in Fig. 3, where CoFe bimetal catalysts are compared with single metal Fe and single metal Co catalysts in ORR and OER electrochemical tests. With single metal Fe and single metal Co catalysts as references, bimetal CoFe catalysts show greater catalytic activity.22) Linear sweep voltammetry (LSV) results in Fig. 3(a) show lower OER onset in the bimetallic catalysts. The Tafel plots shown in Fig. 3(b) identified the bimetallic Co/CoFe@NC (N-doped graphitic carbon) as the most kinetically active with a lowest slope of 49 mV dec−1, even better than that of RuO2 and other catalysts. The cyclic voltammetry (CV) results in Fig. 3(c) and 3(d) give an indication on the electrochemical surface area of the catalysts. By introducing an optimized amount of Fe, Co/CoFe@NC exhibited the largest electrochemical double-layer capacitance (Cdl), suggesting increased electrochemically accessible active sites. Electrochemical impedance spectroscopy (EIS) revealed lower electrode/electrolyte interfacial resistance and faster charge transfer as can be seen from the smallest semicircle in the Nyquist plot (Fig. 3(e)) of Co/CoFe@NC. Figure 3(f) confirms the stability of Co/CoFe@NC as the same OER onset potential is retained after 2000 CV cycles.
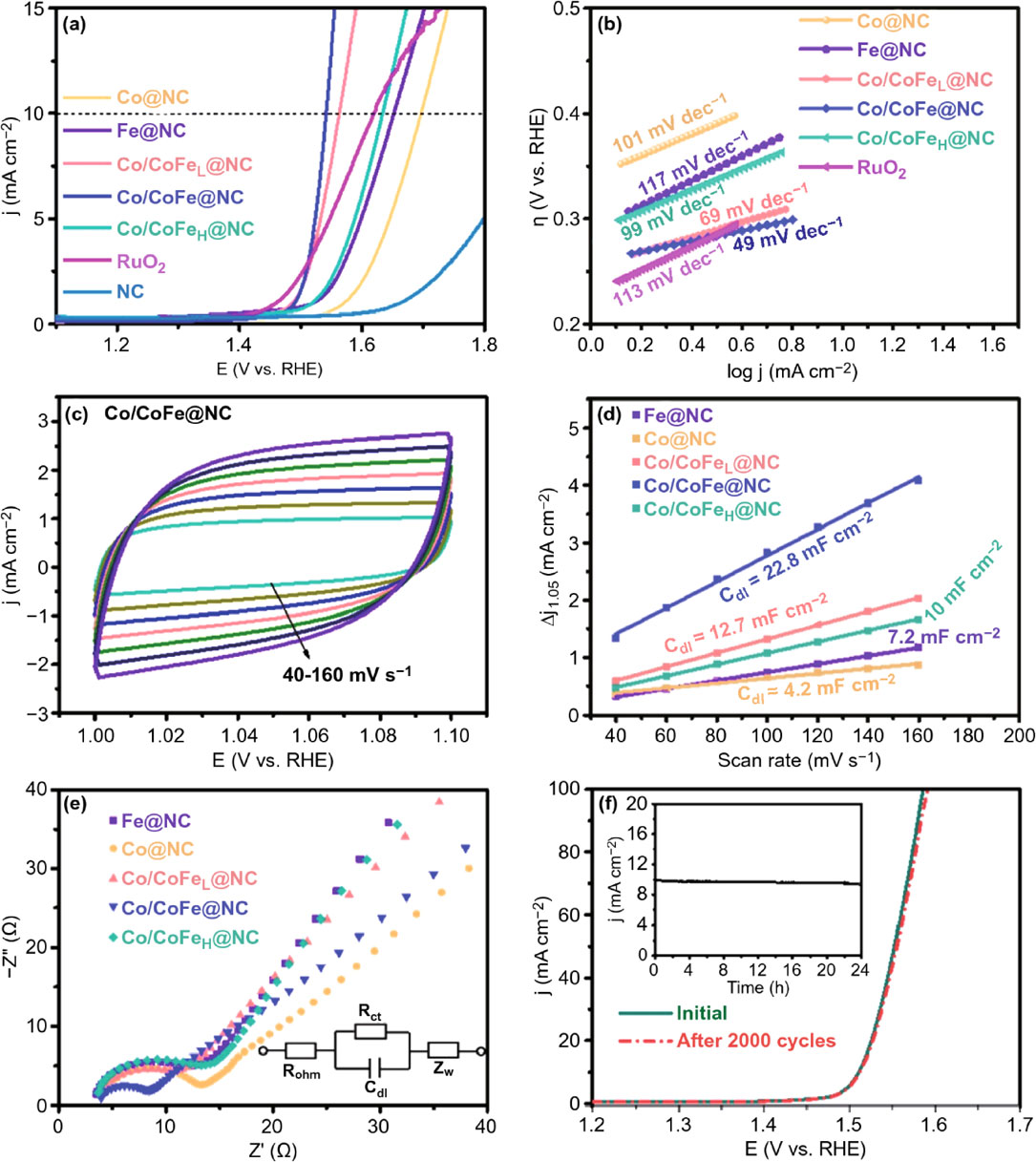
(a) OER polarization curves and (b) corresponding Tafel slopes of the as-prepared catalysts and RuO2 in 1 M KOH. (c) CV curves of Co/CoFe@NC in the double layer region at different scan rates. (d) Current density difference (Δj) at 1.05 V plotted against scan rates of various Co/CoFex@NC samples. (e) EIS spectra recorded at a constant overpotential of 150 mV (inset: equivalent circuit, where Rohm is ohmic resistance, Rct is charge-transfer resistance, Cdl is interfacial capacitance and Zw is warburg diffusion impedance). (f) Polarization curves for Co/CoFe@NC before and after 2000 CV cycles; the inset shows the long-term electrolysis curve of Co/CoFe@NC at an overpotential of 300 mV. (Reproduced from Ref. 22).)
When multiple transition metals in metal oxides are employed, different electronic structures and valence states contribute to greater catalytic activity relative to metal oxides of single metal element.23) Iron and cobalt are common transition metals whose bimetallic oxides have proven electrocatalytic activities.24–27) In one perovskite oxide example, OER overpotential decreased from 0.47 V of CaCoO3 to 0.31 V of CaFe0.5Co0.5O3 by incorporating Fe in the perovskite oxide (Fig. 4).28) In extreme cases such as High Entropy Alloys (HEA) and High Entropy Oxides (HEO), HEA of up to 12 elements of transition metals showed superior catalytic activity and durability under OER and ORR conditions, making them applicable in ZABs.29)
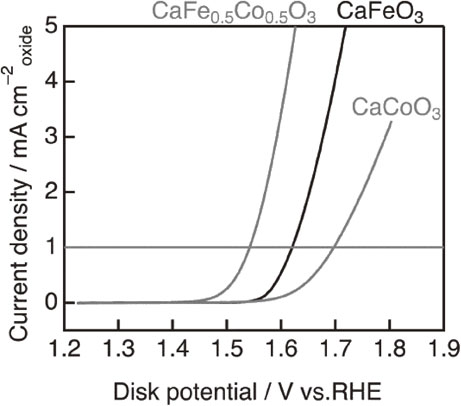
OER linear sweep voltammograms for CaCoO3, CaFeO3 and CaFe0.5Co0.5O3. Electrode potential was swept from 0.3 to 0.9 V vs. Hg/HgO and rotated at 1600 rpm. (Reproduced from Ref. 28).)
While transition metal oxides exhibit catalytic properties as mentioned previously, metal oxides inherently have poor electrical conductivity.30) In general, the catalytic activity of metal nanoparticles tends to increase when their particle sizes are minimized, leading to a larger specific surface area (Fig. 5).10,31–33) Reducing the particle size of metal oxides to the nanoscale enables them to actively engage in electron accepting and donating reactions, despite their inherent insulation properties. Mass activity and specific activity are commonly used metrics to evaluate established catalysts like Pt where morphology and surface area can be accurately determined, and mass is easily quantified.34)

Optimization of Pd nanocrystal size and shape based on (A) turnover frequency (TOF), (B) 2-methyl-3-butyn-2-ol (MBY) transformation rate, (C) selectivity toward 2-methyl-3-buten-2-ol (MBE) at 95% conversion, and (D) transformation rate, or productivity, of MBE. (Reproduced with permission from the Ref. 32). Copyright 2011 American Chemical Society.)
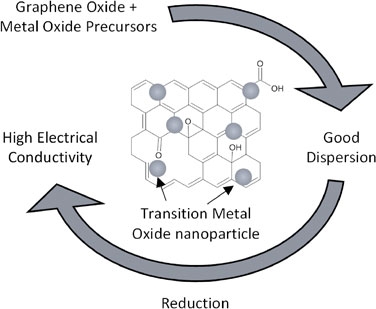
Enhancing the accessibility of catalysts to electrons can also be achieved by depositing them onto electrically conductive substrates, such as carbon nanofibers,19) graphene,35) or reduced graphene oxide (rGO).36–38) (Fig. 6) and other graphene derivatives.17,18,20,21,39) Graphite can be chemically exfoliated to graphene oxide (GO) which, with the oxygen functional groups and structural defects.40,41) Figure 7 shows a summary of the possible oxygen functional groups and structural defects on GO discovered thus far.42) These functional groups are influential in the dispersion of nanoparticles and the formation of thin films.43) GO has limited electrical conductivity on its own, but reduced graphene oxide (rGO), which is obtained by removing oxygen functional groups from GO, exhibits improved electrical conductivity.44–47) Figure 8 shows the increase in electrical conductivity of rGO with increase in reduction temperatures.48) In general, increasing temperature during heat treatment results in the removal of oxygen functional groups and the restoration of the electrically conductive sp2 carbon (C=C bonds) from sp3 carbon.49) GO/MnO2 reduced by hydrazine hydrate (H-rGO/Mn3O4), sodium borohydride (S-rGO/Mn3O4), vitamin C (V-rGO/MnO2) and hydrothermally (T-rGO/MnO2) showed various degrees of increase in electrical conductivity as evidenced by the increase in the integrated area under their respective CV curves (Fig. 9).37) Different heat treatment techniques provide an opportunity to disperse metal oxide precursors in GO and synthesize metal oxide catalysts embedded in rGO through a single thermal decomposition process.50) This approach leverages the dispersion effect of the oxygen functional groups in GO and the resulting electrically conductive rGO as the final product.51,52)
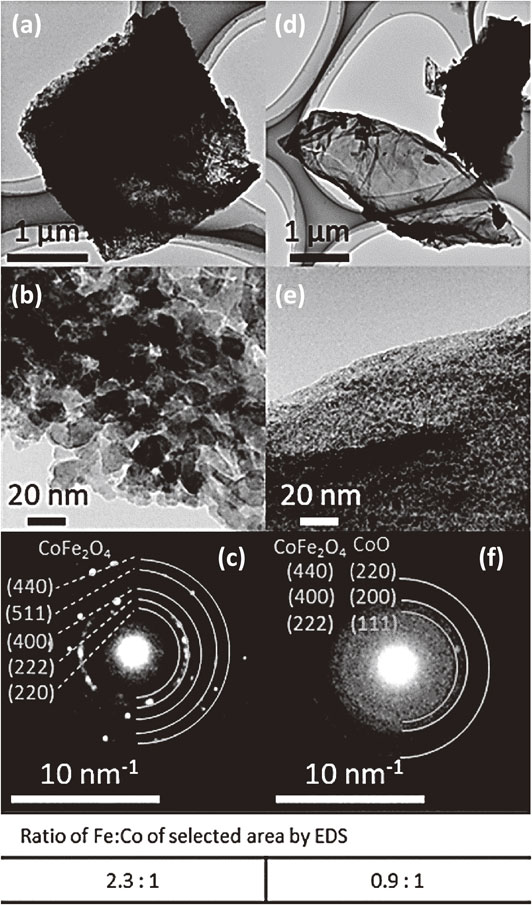
Transmission electron microscopy (TEM), selected area electron diffraction (SAED), and energy dispersive x-ray spectroscopy (EDS) results of FeCo oxide particles on rGO (FeCo-rGO). (a) and (d) TEM images of FeCo-rGO at low magnification. (b) TEM image of distinct particles magnified from (a). (c) SAED pattern of particles in (b) assigned to spinel oxide, CoFe2O4. (e) TEM image of an area where particles could not be resolved magnified from (d). (f) SAED pattern of particles in (e) could be assigned to either CeFe2O4 (yellow indices) or CoO (blue indices). Fe:Co ratios of both areas selected for (c) and (f) were verified by EDS. (Reproduced with permission from Ref. 36). Copyright 2022 The Royal Society of Chemistry.)

Proposed structure of graphene oxide layer. (Reproduced from Ref. 42).)

Left: plot showing electrical conductivities of reduced graphene oxide against reduction temperatures, together with the thermal gravimetric analysis (TGA) curve. Right: the table summarizes electrical conductivity against the reduction temperature. (Reused with permission from Ref. 48). Copyright 2015 American Chemical Society.)

Cyclic Voltammograms of GO/MnO2, MnO2, rGO, T-rGO/MnO2, V-rGO/MnO2, S-rGO/Mn3O4 and H-rGO/Mn3O4 electrodes carried out at 10 mV·s−1. (Reproduced from Ref. 37).)
Industrially, ZABs are designed with the electrolyte under constant circulation.53) This allows active materials to be stored externally thus allowing theoretically unlimited capacity scalability.54) In this zinc-air flow battery configuration, current densities as high as 100 mA cm−2 are tested.55) Hybridizing transition metal oxides with reduced graphene oxide has been shown to improve battery cycling performance under such high current density operations (Fig. 10).36,56) The improve in performance is thought to be increased catalyst activation due to the fine and uniform dispersion of transition metal oxides on the surface of rGO particles.36)

Improved cycling performance of ZAB cells with FeCo oxide catalysts hybridized with reduced graphene oxide. (Reproduced from the Ref. 36). Copyright 2022 The Royal Society of Chemistry.)
In this article, general ZAB chemistry and the role of catalysts in these cells are reviewed. For high current density ZAB operations, catalyst activation is necessary and the most common methods are miniaturization of catalysts to increase specific surface area and dispersion of catalysts on electrically conductive substrate to improve electrical access. Most notably, recent works have improved cycling performance under 100 mA cm−2 operations by hybridization with carbon derivatives such as rGO.
Partial financial support by Hokkaido University is gratefully acknowledged. Also partial financial supports from Young Research Acceleration Project of Hokkaido University, Grant for Basic Science Research Projects from the Sumitomo Foundation, and the Kurata Grant awarded by the Hitachi Global Foundation for MNT are acknowledged. MTN also thanks for the financial support from Grant-in-Aid for Scientific Research (C) from JSPS. TY thanks partial financial supports from Grant-in-Aid for Scientific Research for priority area (21H00138) and JST aXis-B. WJS appreciates the financial support from the Mitsubishi UFJ Trust Scholarship Foundation for his stay in Sapporo. This work was supported by JST SPRING, Grant Number JPMJSP2119.