2023 Volume 64 Issue 11 Pages 2637-2642
2023 Volume 64 Issue 11 Pages 2637-2642
In former studied, authors have already succeeded to get chill-free spheroidal graphite iron castings of φ35 × t5.4 mm indicator sample and automobile knuckle, using permanent mold in as-cast condition. The process of melting, molten treatment and pouring were theoretically designed considering both the reduction and increase of free nitrogen (NF), but it was not analytically proved by actual data yet. In this study, the amount of NF was analyzed using chill samples, which were taken at critical points like melting and molten treatment. As the results, the processes which reduce and increase NF were made clear. The increasing NF was occurred on the process during heating up, carbon addition and temperature slightly increased at near the TEC. The decreasing NF was occurred the process of alloy addition, during super-heating and temperature decreased at under the TEC. Furthermore, as the amount of molten metal decarburization increased, the variation of NF changed from adsorption to denitrification.
In early 1970s, permanent mold (PM) casting had been studied vigorously in all over Japan, especially, Kansai and Tokai districts. However, chill free casting was not achieved on spheroidal graphite iron (SGI) and was needed heat treatment to graphitize chill. As the reason, it is considered that the mechanisms of graphite spheroidization and graphitization (conversely, chill formation) were not understood enough and were in chaotic at that time. As the kind of reports, technical book entitled PM casting in cast iron1) was published. After the big project, Y. Lee2) and T. Kitsudo3) had studied about chill free PM casting in SG iron castings at different timing individually and moreover separate institution. They succeed to have chill free condition in Φ10∼30 mm bar for each, but unfortunately, the clear factor of chill formation as the mechanism could not be confirmed. For example, Y. Lee concluded that acid soluble nitrogen (Nsol) caused chill and less Nsol brought full graphite structure. Y. Lee developed own theory to avoid chill formation. However, Nsol contains free nitrogen (NF), AlN, Mg3N2 and Si3N4. NF could not be analyzed alone at that time. Therefore, his theory was difficult to understand for researchers in laboratories and engineers in foundries. Moreover, T. Kitsudo reported phenomena on full graphite structure by PM casting but could not show the mechanism. Therefore, there was a lack of reproducibility to have chill free casting in as-cast condition at other parties. Currently, there are foundries who adopt PM casting method for DCI castings however they do not clear chill yet and need heat treatment even now. Looking around the world, researchers4) at laboratories reported no chill in larger size of sample castings like Φ40∼60 mm. However, almost no foundry can get chill free castings in as-cast, and they need to take heat treatment so far.5)
H. Itofuji, et al.6) reported recently that there was good correlation between NF in molten metal and the tendency of chill formation, and if NF can be controlled less enough, chill formation can be avoided. In the paper, the stable production of SGI castings by arc furnace melting, which had been considered extremely difficult to avoid chill, was introduced with the fundamental mechanisms. NF is atomic nitrogen, and NF was calculated by total nitrogen content (NT) minus inclusive nitrogen content (NI). NF has been also used in the steel sector for production regulation since 2017.7) H. Itofuji et al.8) had also suggested that this NF might promote ledeburite (γ+Fe3C) solidification by forming iron carbonitride (Fe3(CN)). In their study, thin wall PM of φ37 × t3.5 mm, which is normally used as chill sample castings for spectrometer analysis, was used for chill free sample castings under imaging above theories. The chill free automobile knuckles were also obtained in as-cast condition.8) They stated that the results came from understanding mechanisms which were site theory9,10) as graphite spheroidization and NF theory as chill formation. However, the critical value of NF in PM casting was not shown yet. It is forecast the severer control of NF might be needed for PM casting in practice than that for conventional sand casting. Furthermore, the upper limit of NF must be known for each size of castings in the stable mass production. In this study, the trend of increase/decrease of NF in each melting process and relationship between decarburization content and change of NF content was founded by analyzing the NF content in each melting process. The results can be useful to design suitable process.
A 30 kg high frequency electric furnace inserted magnesium crucible was used for melting base iron. The raw materials that were shown in Table 1 were mix. The designed chemical composition of molten iron was shown in Table 2. Hypereutectic composition of 3.60 mass% C and 3.30 mass% Si was selected. Chilled samples were taken for chemical analysis and were analyzed using spectrometer. Furthermore, C was also analyzed by the method of oxygen air flow combustion - infrared absorption. Spheroidizing agent and inoculant, which contain low NT, were selected for molten treatment. Spheroidizing agent was developed for PM casting.11) Their chemical composition is shown in Table 1.


To minimize nitrification from air, Plunger was used for spheroidizing treatment in furnace. During the treatment, air was cut off using double lids. Mg-treated molten iron was inoculated during tapping, and so-called stream inoculation was conducted. The temperature of molten metal for each melting process was controlled the base on CO/SiO2 critical equilibrium temperature (TEC)9,10) calculated by Oelsen’s formula from C and Si contents in base molten iron. The molten metal temperatures for super heat process, holding process and spheroidization were controlled to target at over TEC + 65 K, TEC ± 5 K and TEC − 20 K, respectively. The TEC value is obtained using (1).
| \begin{equation} T_{\text{EC}}\ (\text{K}) = 27{,}486/\{-{\log}([\text{Si}]/[\text{C}^{2}]) + 15.47\} \end{equation} | (1) |
The molten metal after treatment was poured in PM shown Fig. 1 for samples of microstructural analysis. Water base coat and acetylene soot was coated on cavity surface of PM. The samples for spectrometer analysis were poured in same type of PM without coating. The time-temperature schedule and sampling points from melting to pouring are shown in Fig. 2.

Shape and dimension of permanent mold.
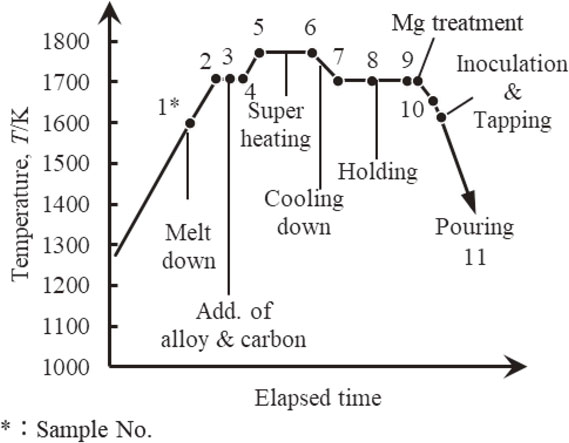
Sampling points from melting to pouring.
The confirmation of chill and the analysis of spheroidal graphite structure were conducted on vertical cross section. The graphite grain size was subject over 1 µm in analysis of spheroidal graphite because graphite grain size was ultra-fine. The samples for analysis were taken at 11 inflection points from melt down to casting Fig. 2. The flow chart for quantitative method of NF was shown in Fig. 3.11,12)

Analysis procedure of nitrogen morphology in chilled iron samples.
The results of chemical composition analysis at all sampling points are shown in Table 3. Temperature at every sampling point is also in Table. Macro and microstructure in PM sample castings are shown Fig. 4. The chill was not observed at all. The results of graphite structural analyses are shown Table 4. The graphite nodule was extremely fine, and the average of graphite diameter was 8 µm. The maximum nodule number were 2523 count/mm2. The nodularity was 90%.


(a) Ingot and (b) microstructure of SG after permanent mold casting. (3% Nital Ecth)

The relationship among molten iron temperature, amount of nitrogen morphologies and chemical composition in change are shown in Fig. 5. The factors which are considered to influence the quantitative change of nitrogen morphologies are summarized in Table 5. The significant difference of nitrogen variation was considered difference of 2 mass ppm or more, because that scattering of the analysis value was less than 2 mass ppm. The NT remained around 40 mass ppm with small variation. However, the variation of NI was remained between 20 mass ppm and 42 mass ppm, and the variation of NF was remained between 0 mass ppm and 20 mass ppm. The variation of NI and NF were larger than NT. This suggests that the main variation of nitrogen were NI formation and discharge and NF absorption and discharge. The Si, Al and Ca was variated significantly in the results of chemical composition analysis when adding alloy process, spheroidization process and inoculation process. The C was gradually decreased from 3.60 mass% to 3.39 mass% after adjusting chemical composition. The Mn was increased 0.01% when adjusted chemical composition. The increasing of Mn was presumed that contamination from reduced slag on furnace wall, because alloys containing Mn was not used when adjusted chemical composition. In this study, no correlation had been obtained clearly between each nitrogen content, molten metal temperature and chemical composition. Therefore, primary factor of NI and NF variation was guessed that factors other than molten metal temperature and chemical composition was also included. The difference from the next process on nitrogen contents was used as the number of variations and the number of variations were arranged.

The relationship among (a) temperature, (b) nitrogen amount, (c) C, Si amount, (d) Mn, S, Mg and (e) P, Al, Ca amount.

The processes which made amount of NF increase were heat up ① and ②, addition of graphite, holding ① and inoculation. NT showed slight change and almost same through the processes. NT increased slightly when NF increased greatly and after Mg treatment. An increase in NF is observed in heat up ① and ② and holding ①, even though no additives are used. This suggests that the source of NF adsorption and nitrification is from the atmosphere. The decrease in NI is due to the stirring effect of the induction current and turbulence during tapping, which causes NI to rise to the surface of the molten iron. The decrease in NI is due to the stirring effect of induced current and turbulence during tapping. NF showed almost no change at the process of Mg treatment. Two reasons are considered for this phenomenon. The first is that molten surface might be cut off completely from air during the treatment by double covers.13) The second is that Mg alloy with low NT was used for treatment. NF increased when inoculation was conducted. As the reason, the exposure of stream during tapping and the turbulence of molten iron in ladle are considered. It is necessary to improvement this process since it is almost the final stage for influencing the microstructural quality of castings.
The other processes that NF decreased were adding alloy, super heating, and holding ②. These processes were observed that the NI largely increased and the difference between NT and NI smaller. After alloy addition, NI increased largely because Al and Ca. Al and Ca in alloys were strong affinity with nitrogen and easy to form nitrides.14) The increasing in NI and the decreasing NF during super heat, it was guessed that the effect of reducing MgO lining by C in molten iron and denitrification by reduced Mg. The equations for the reaction were shown below.
| \begin{equation} \text{2C} + \text{SiO$_{2}$} \to \text{Si} + \text{2CO}\uparrow \quad \text{Reduction of SiO$_{2}$ by C} \end{equation} | (2) |
| \begin{align} \text{C} + \text{MgO} &\to \text{Mg} + \text{CO}\uparrow \\ &\quad \text{Reduction of MgO lining by C} \end{align} | (3) |
| \begin{equation} \text{3Mg} + \text{2N} \to \text{Mg$_{3}$N$_{2}$}\quad \text{Bonding Mg and N} \end{equation} | (4) |
Holding ① and ② were molten metal temperature holding processes near TEC, but the behavior of nitrogen morphology is different. The phenomenon was considered that effect of molten metal stirring by induced current. The temperature of holding ① increased 13 K and the temperature of holding ② decreased 58 K. In other words, the holding ① was considered to have more molten metal stirs than holding ②. Holding ① is considered to be due to the absorption of nitrogen from the atmosphere into the molten metal due to discharge of nitrides by agitation of the molten metal and increased contact opportunity with the atmosphere. Holding ② is thought to have suppressed the absorption of nitrogen by reducing contact with the atmosphere and increased NI as the NF absorbed in holding ① became nitride.
The relationship between amount of decarburization and amount of NF variation was showed in Fig. 6. The variations in decarburization and NF represent the differences from process. The absorption of NF (ΔNF) was decreased as the amount of decarburization increased. Furthermore, the tendency was changed by the degree of decarburization. The turning point was about 0.05%. The decarburization is one of phenomena by CO boiling (formula (1)) in molten iron.15) It is known that a method of denitrification by CO boiling in refining process of cast steel.16) These suggest that phenomenon of NF variation with the amount of decarburization was affected of CO gas. The CO boiling was considered that occurred at TEC or more. No. 6-7 was furnace cool process of molten temperature and considered that included the decarburization and oxidation reaction. The oxidation reaction was considered that superiority in No. 7-8, because the molten temperature was held in below the TEC. The decarburization reaction was considered that superiority in No. 5-6, because the molten temperature was held in above the TEC. Therefore, the relationship between decarburization amount in these three processes was considered appropriate. The No. 4-5 was heating process in temperature of above TEC and decarburization reaction was considered superiority. However, the decarburization amount was least. The No. 4-5 was added alloy in the process immediately before, and the oxygen content that needed to decarburization reaction was low that showed in Table 3 and Fig. 5. Therefore, No. 4-5 was considered that had the low decarburization amount because the decarburization reaction was difficult.

Relationship between ΔNF and amount of decarburization.
In this study, the variation factor in the amount of NF was investigated by direct analysis of samples taken at each melting process when chill-free PM casting in spheroidal graphite iron was succeeded. As the results, the followings became clear.