2023 Volume 64 Issue 11 Pages 2568-2574
2023 Volume 64 Issue 11 Pages 2568-2574
In this study, microstructure and phase transformation behavior in Fe20Co20Ni20Cr20B20−xSix alloys prepared by the mechanical alloying (MA) method were investigated by X-ray diffraction (XRD) measurements, scanning electron microscopy, transmission electron microscopy, and differential scanning calorimetry (DSC). The Fe20Co20Ni20Cr20B20−xSix alloys prepared by the melt-spinning method were composed of FCC and compounds, and the FCC and BCC phases predicted by the valance electron concentration parameter were not formed. However, alloy powders prepared by the MA method were revealed to be composed of the FCC and BCC phases. Small amounts of unreacted pure B and Si particles were observed in alloy powders with high and low B content, respectively. XRD and DSC measurements revealed that the BCC phase in MA powder disappeared, and compounds were formed by heating up to 700°C. Especially the compound formation temperature was higher than that of the same alloy prepared by the melt-spinning method, suggesting that the thermal stability of the alloy powders prepared by the MA method was higher than that of the alloy ribbons prepared by the melt-spinning method.
Conventional alloys are designed based on the concept of selecting one or two principal constituent elements (such as Cu and Ti alloys). High-entropy alloys (HEAs) are new concept alloys without principal constituent elements and composed of five or more elements with equal or near-equal atomic ratios.1) HEAs have attracted the attention of researchers around the world because they exhibit excellent properties,2–5) such as outstanding strength, fracture toughness, and corrosion resistance, owing to the contribution of the four core effects:1) high entropy, sluggish diffusion, lattice distortion, and cocktail effects.
HEAs were initially investigated only for alloys composed of BCC and/or FCC phases.6–8) However, more recently, HEAs composed of HCP phase9) and multi-phase HEAs containing compounds10,11) have been reported, and the definition of HEAs is expanding. The HEAs reported so far have been prepared by various methods, including arc melting,12) high-frequency induction melting,13) Bridgeman method,14) rapid solidification (like melt-spinning),15) and mechanical alloying (MA).16) The MA method is used for alloying powders by repeated mechanical milling and mixing without melting. The MA method enables uniform alloys to be obtained from materials with widely different melting points and densities.17) In addition, MA method can impart high mechanical energy to the materials, which can form different microstructures compared with alloys solidified from the liquid phase. Furthermore, alloys prepared by MA method are expected to exhibit new properties. Many researchers have reported HEAs prepared by the MA method. For example, Yu et al. reported that the hardness of CoCrFeCuNi and CoCrFeMnNi alloys prepared by MA combined with high-pressure sintering (HPS) was 494 Hv and 587 Hv, respectively, which was higher than that prepared by the casting method.18) Mohanty et al. reported that equiatomic AlCoCrCuFeNi alloy prepared by MA method exhibited a hardness of 92 Hv, which increased to 816 Hv when combined with sintering.19)
Our group has investigated Fe20Co20Ni20Cr20B20−xSix alloys solidified at various cooling rates and reported their constituent phases and phase transformation behavior.20,21) These alloys solidified at a cooling rate of 5.3°C/s were composed of FCC, Cr2B, and Cr3Ni5Si2 phases. The Fe20Co20Ni20Cr20B15Si5 alloy solidified at a cooling rate of 7440°C/s was found to be composed of nanoscale FCC and Cr2B phases, whereas the others were found to be composed of FCC, Cr2B, and Cr3Ni5Si2 phases. In alloys solidified at 2.4 × 105°C/s, an amorphous phase was formed in alloy ribbons with a B content of above 7.5 at%.
HEAs with high metalloid content are reported to exhibit excellent mechanical properties, avoiding the commonly expected trade-off between ductility and strength.22) In addition, HEAs with metalloid elements are expected to have potential as a functional material.23) To identify new properties of alloys, it is critical to investigate their constituent phases, as well as the effects of various preparation methods on the constituent phases in the alloys. However, to our knowledge, the effects of the preparation method on the constituent phases in HEAs with high metalloid content have not been statistically investigated. Therefore, this study aimed to evaluate the phase formation and phase transformation behavior during heating in Fe20Co20Ni20Cr20B20−xSix alloys with high B and Si content prepared by the MA method, and to compare them with the same alloys prepared by the melt-spinning method.
High-purity Fe, Co, Ni, Cr, B, and Si powders (<100 mesh) were used as starting materials. The powders were weighed to match each composition ratio and sealed with zirconia balls (ϕ = 5 mm) in a zirconia pod under a purified argon atmosphere. The weight ratio of the powder-to-balls was 1:10. The sealed pods were set in a planetary ball mill (PULVERISETTE 7 premium line, Fritsch), and the rotation speed was set at 400 rpm. All specimens were milled for up to 24 h. Milling was paused for 10 min after every 10 min of milling to avoid overheating the powder.
Phase identification was performed using X-ray diffraction (XRD) measurements (MiniFlex600, Rigaku), and powder morphology was observed using scanning electron microscopy (SEM; JCM-6000, JEOL). The microstructural observation was performed using transmission electron microscopy (TEM)/Scanning-TEM (STEM; JEM-F200, JEOL) with energy dispersive X-ray spectroscopy (EDS; JED-2300T, JEOL). The specimens for TEM investigation were mounted in epoxy resin (G2, Gatan), polished using a tripod polisher, and finished using a focused ion beam (FB-2000A; Hitachi). Phase transformation behavior during heating was investigated using differential scanning calorimetry (DSC; Thermo plus EVO2 DSC 8230, Rigaku).
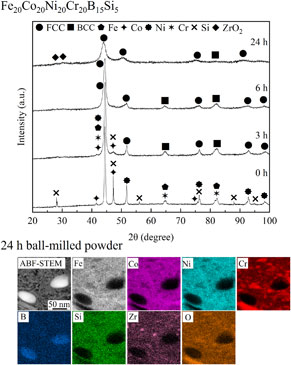
Figure 1 shows the XRD patterns of Fe20Co20Ni20Cr20B15Si5 (15B5Si) alloy powders with different milling times. The XRD pattern of the powder without MA milling showed peaks corresponding to FCC, BCC, HCP, and diamond_Si phases. However, the peak of B is not observed in XRD measurement results, probably because the scattered X-ray was absorbed by the Fe elements.24) The peaks of FCC correspond to Ni, the peaks of BCC correspond to Fe and Cr, and the peaks of HCP correspond to Co. The atomic radii of the metal elements are nearly equal, indicating that the peaks of Fe and Cr overlap. The peak intensity decreased in the XRD pattern after 3 h of milling, indicating that the powder particles have been refined by MA milling. The XRD pattern of the powder after 6 h of milling showed that the peaks corresponding to Co and Si have wholly disappeared. After 24 h of milling, the XRD pattern only exhibited peaks corresponding to FCC and BCC phases, the peak intensity further decreased, and the peak width broadened. Furthermore, the intensity of the peak corresponding to the BCC phase was very weak compared to the peaks corresponding to the FCC phase. This is probably because of the crystal refinement and lattice distortion caused by the MA milling. A shift of the peak to the low-angle side was also observed, suggesting that the lattice constant increased because of the formation of the solid solution by MA milling. The occurrence of contamination during MA milling was reported by Joo et al. when using zirconia pod and balls.25) In this study, a peak corresponding to ZrO2 was also observed, suggesting the presence of zirconia contamination.

XRD patterns of Fe20Co20Ni20Cr20B15Si5 alloy powder milled for different times.
Figure 2 shows the XRD patterns of Fe20Co20Ni20Cr20B20−xSix alloys after 24 h of milling. The milling time was limited to 24 h in this study because the identification of phases becomes difficult after a long milling time due to the overlap of the peaks corresponding to ZrO2 with peaks of other phases in all compositions. Peaks corresponding to the FCC phase and weak peaks corresponding to BCC phase were observed, and peaks corresponding to Si in an unreacted state were also identified. Because milling was only performed for up to 24 h in this study, it cannot be determined whether the presence of unreacted Si is due to the solution limit being reached or insufficient time for MA milling. Furthermore, weak peaks corresponding to ZrO2 were observed, and decreased with increasing Si content, which can be attributed to the decrease in the amount of ZrO2 contamination. B is the hardest of the constituent elements, even harder than ZrO2, suggesting that the contamination of ZrO2 increases with higher B content. The XRD patterns of 15B5Si and Fe20Co20Ni20Cr20B12.5Si7.5 (12.5B7.5Si) prepared with the melt-spinning method showed peaks corresponding to the FCC and Cr2B phases, and the XRD patterns of the other three compositions (x = 10, 12.5, 15) exhibited peaks corresponding to the FCC, Cr2B, and the Cr3Ni5Si2 phases.20,21) However, the XRD patterns of the MA powders did not show any peaks corresponding to the compounds.

XRD patterns of Fe20Co20Ni20Cr20B20−xSix alloy powders milled for 24 h.
Figure 3 shows secondary electron images (SEIs) of MA powders after 24 h of milling. According to the SEIs for each composition, it was observed that the grain size of the powder particles with higher B content was finer than that of the powder particles with lower B content. Flake-like particles were observed in compositions with B content lower than 10 at%. The process of MA was reported to proceed in multiple stages.26) The raw material particles are flattened in the initial stage, and the flattened particles are repeatedly cold welded to each other. Then equiaxed-shaped particles are subsequently formed, and finally, the particles are homogenized through random cold welding. Moreover, Fogagnolo et al. reported that the presence of brittle particles during MA method could lead to their embedding within ductile particles, resulting in forming composite particles that enhance the milling process.27) The hardness of B is higher than that of the other constituent elements of Fe20Co20Ni20Cr20B20−xSix alloys and zirconia. Flake-shaped powders were not observed from SEIs of powders with higher B contents, suggesting that B promotes the milling. Furthermore, the amount of contamination from the milling medium increased with increases in B content, suggesting that the synergistic effects of B and zirconia may have promoted the milling process. Relatively large particles were observed in some compositions. In the MA process, it has been reported that larger particles are formed by the agglomeration of smaller particles.25)

SEIs of the Fe20Co20Ni20Cr20B20−xSix powders milled for 24 h. (a) x = 5, (b) x = 7.5, (c) x = 10, (d) x = 12.5, and (e) x = 15.
Figure 4 shows the bright field (BF)-TEM images and selected area electron diffraction (SAED) patterns for 15B5Si, Fe20Co20Ni20Cr20B10Si10 (10B10Si), and Fe20Co20Ni20Cr20B5Si15 (5B15Si) alloy powders after 24 h of milling, and Fig. 5 shows the EDS elemental maps. These elemental maps were taken with the characteristic X-rays of Fe-Kα, Co-Kα, Ni-Kα, Cr-Kα, Si-Kα, Zr-Lα, and O-Kα. Cr-rich, Fe, Co, and Ni-rich regions were observed in all compositions, and correspond to BCC and FCC phases, respectively. B-rich regions were also observed in 15B5Si, Si-rich regions were observed in 5B15Si, and both regions were observed in 10B10Si. In addition, Zr-rich regions corresponding to zirconia were observed. Interestingly, the EDS analysis did not detect any other elements, including O, in either the B- or Si-rich region, indicating the presence of B and Si in a pure elemental state. Ipus et al. reported that B in an unreacted state could be seen in Fe–Nb–B alloy powders despite 400 h of the MA process.28) Liu and Chang reported the formation of B2O3 in Fe–(Co,Ni)–Zr–B alloy powder using the MA process.29) Although the alloy powders prepared in this study exhibited the presence of B and Si, no evidence of oxide formation was observed. This result is probably because of the oxidation suppressed by filling the pod with purified argon.

(a), (c), and (e) BF-TEM images of Fe20Co20Ni20Cr20B20−xSix alloy powders milled for 24 h, (a) x = 5, (c) x = 10, and (e) x = 15. (b), (d) and (f) SAED patterns were obtained from the areas marked with circles in (a), (c) and (e).

ABF-STEM images and EDS elemental maps of Fe20Co20Ni20Cr20B20−xSix alloy powders milled for 24 h. (a) x = 5, (b) x = 10, and (c) x = 15.
The interplanar distances were calculated from the SAED patterns obtained from the areas marked with circles in Fig. 4(a), (c), and (e) and corresponded to the interplanar distances of BCC or FCC phases. A weak ring pattern corresponding to ZrO2 was also observed in 10B10Si. Although it has been reported that B occupies the substitutional site in case of dissolution in the BCC phase30) and the simultaneous occupation of the substitutional and interstitial sites in the case of dissolution in the FCC phase,31) the amount of B dissolved in B—transition metal binary systems is reported to be extremely limited. However, the XRD and TEM results revealed that no compounds were formed in the powder prepared by the MA method. These results suggest metastable phases with a high energy hierarchy were formed during the MA method, successfully obtaining FCC and BCC phases with high B content. The XRD measurement results showed that the peaks corresponding to BCC were very weak. However, TEM observation revealed that a relatively large amount of BCC solid solution was formed.
The constituent phases formed in HEAs can be predicted using the parameter method. The parameter method statistically organizes the results of previous studies to predict the formation tendency and structure of the solid solution.32–34) The valance electron concentration (VEC) parameter is often used to predict the crystal structure of HEAs. VEC is the number of electrons per atom in the valence band. Guo et al. found that HEAs with VEC higher than 8.0 will form the FCC phase, HEAs with VEC lower than 6.87 will form the BCC phase, and HEAs with VEC in the range of 8 to 6.87 will form the FCC and BCC phases.34) The values of VEC for 15B5Si, 12.5B7.5Si, 10B10Si, 7.5B12.5Si, and 5B15Si were 7.25, 7.28, 7.30, 7.33, and 7.35, respectively. This result indicates that Fe20Co20Ni20Cr20B20−xSix alloys have a tendency to form FCC and BCC phases. According to the XRD and TEM results, the Fe20Co20Ni20Cr20B20−xSix alloy powders prepared by the MA method were composed of the structure predicted by the VEC parameter method. However, neither the high-frequency melting nor the melt-spinning method could form the predicted structure in Fe20Co20Ni20Cr20B20−xSix alloys. HEAs have been prepared by various techniques, and some alloys have been reported to exhibit structures that differ from those predicted by the parameter method. For example, He et al. reported that the (FeCoCrNiMn)100−xAlx (x = 0–20 at%) alloys were predicted to form FCC and BCC phases in all compositions according to the VEC parameters. However, part of the alloys prepared by the casting method was composed only of the FCC phase.35) On the other hand, Cheng et al. reported that FeCoCrNiMnAlx (x = 0–1) alloys prepared by the MA method were composed of FCC and BCC phases in all compositions as predicted by the VEC parameter.36) The present experimental results and previous reports suggest that the currently used VEC parameter does not sufficiently consider the effects of the preparation method on the constituent phases in the alloys.
Figure 6 shows the DSC heating curves up to 700°C of MA powders after 24 h of milling. According to the DSC measurement results, two exothermic peaks were identified for MA powders other than 5B15Si. This exothermic behavior is similar to that of the same alloys solidified at a cooling rate of 2.4 × 105°C/s,20) which were composed of an amorphous phase. However, the exothermic peaks were shifted to higher temperatures than were those for amorphous alloy ribbons. Figure 7 shows the XRD patterns of each powder after DSC measurements. The XRD pattern after the DSC measurements showed that the peaks corresponding to the BCC phase disappeared, and the peaks corresponding to the compound were observed. The XRD patterns after DSC measurements of 15B5Si and 12.5B7.5Si showed peaks corresponding to Cr2B that were identified after heating to 700°C. In other compositions, peaks corresponding to Cr3Ni5Si2 were also observed in addition to Cr2B. Although the identified phases after heating to 700°C were the same as those of the alloy ribbons solidified at a cooling rate of 7440 and 2.4 × 105°C/s, the phase transformation behavior was different.

DSC heating curves of Fe20Co20Ni20Cr20B20−xSix alloy powders milled for 24 h.

XRD patterns of Fe20Co20Ni20Cr20B20−xSix alloy powders milled for 24 h after DSC measurements.
To investigate the phase transformation behavior during heating in more detail, XRD measurements were performed on samples that were heated to just above the first exothermic peak using DSC. Figure 8 shows the XRD patterns of 15B5Si powder heated to 616°C and 700°C. The XRD pattern of the 15B5Si powder heated to 616°C showed disappearance of the BCC peak, suggesting that the BCC phase transformed into the FCC phase. In addition, peaks corresponding to Cr2B were observed in the XRD pattern of 15B5Si powder heated to 700°C, suggesting that Cr2B was formed. Because ZrO2 is an inert material, no change was observed after heating to 700°C. A qualitative comparison was made between the phase transformation behavior observed on MA powders during heating and that obtained by thermodynamic equilibrium calculations. According to thermodynamic equilibrium calculation results, the 15B5Si alloy shows a tendency for the BCC phase to disappear above 500°C, and the mole fractions of FCC and Cr2B phases exhibit a tendency to increase. The phase transformation behavior of the alloy powder prepared by the MA method revealed in this study generally corresponds to the thermodynamic calculation results. Although the thermodynamic equilibrium calculation results indicate an equilibrium state, the phase transformation behaviors were qualitatively consistent with those observed in the MA powders.

XRD patterns of Fe20Co20Ni20Cr20B15Si5 alloy powders milled for 24 h in as-milled state and after DSC measurements.
Our group has reported that alloy ribbons composed of the amorphous phase exhibited a two-step crystallization process during heating to 700°C. Figure 9 shows the DSC curves of the 15B5Si amorphous alloy ribbon20) and alloy powder milled for 24 h, and the relationship between the exothermic onset temperatures and Si content for Fe20Co20Ni20Cr20B20−xSix alloy powders and amorphous alloy ribbons.20) Part of amorphous phase in the alloy ribbon first transformed into the BCC phase with increasing temperatures and then into the FCC phase with a further increase in temperature.21) In addition, compounds are formed at the same time as the appearance of the FCC phase. Although the as-spun amorphous alloy ribbons and MA-milled alloy powders exhibit different constituent phases, the final constituent phases are the same after heating to 700°C. Compounds are formed when both the alloy powder and alloy ribbon pass through the exothermic peak on the high-temperature side. However, the peaks of the MA powder were shifted to the high-temperature side, indicating that the compound formation temperatures of the MA powder are higher than those of the ribbon. Oleszak et al. reported that the crystallization temperature of amorphous alloys produced by the MA method is higher than that of the same alloys prepared by the melt-spinning method.37) The results of previous reports and the present study suggest that present HEAs prepared with the MA method exhibit improved thermal stability. Moreover, comparing the Tx2 of Fe20Co20Ni20Cr20B20−xSix alloy ribbons composed of amorphous phase with that of Fe20Co20Ni20Cr20B20−xSix alloy powders, the effects of Si content on Tx2 show different trends for the ribbons and powders. This is because the constituent phases during compound formation are different, i.e., the compounds in the alloy ribbons were formed from the BCC or amorphous phases, while the compounds in the alloy powders were formed from the FCC phase.
In this study, we evaluated the constituent phases and phase transformation behavior of Fe20Co20Ni20Cr20B20−xSix alloys prepared by MA method and compared them with those for alloys fabricated by the melt-spinning method. The effect of the preparation method on the microstructural formation in HEAs with high B and Si contents was clarified, thereby providing a guideline that can contribute to the design of HEAs with high metalloid element contents. The following conclusions were obtained in this study.
The authors are grateful to Mr. Noboru Wakayama of the Center for Instrumental Analysis, Kyushu Institute of Technology, for help with the TEM observation.