2023 Volume 64 Issue 2 Pages 540-547
2023 Volume 64 Issue 2 Pages 540-547
The influence of metal cations on corrosion behavior of aluminum alloy (AA2024-T3) in model freshwater was examined by immersion experiments, surface analysis, and electrochemical measurements. After long-time immersion, the corrosion rate of AA2024-T3 decreased as the corrosion indicator Y increased, and the corrosion rate of the alloy was reduced by Zn2+. The specimen immersed in Zn2+ containing solution showed less corrosion products which were observed by scanning electron microscopy (SEM). The results of X-ray photoelectron spectroscopy (XPS) and Auger electron spectroscopy (AES) showed that Zn2+ attaches to aluminum alloys and forms protective layers. Electrochemical impedance spectroscopy (EIS) results suggested that Zn2+ reduced the area of defects in the passive film.
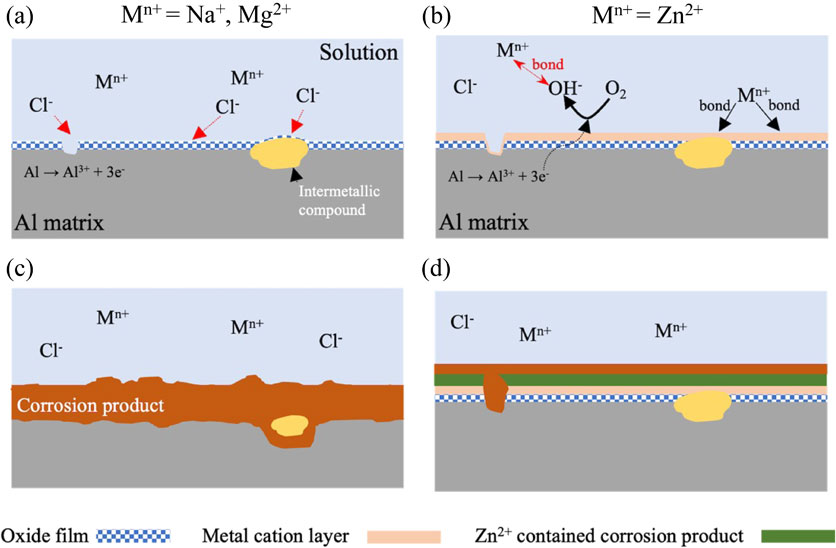
Fig. 9 Corrosion mechanism of AA2024-T3 in model freshwater containing metal cations with (a), (c) small Y, and (b), (d) large Y.
Aluminum alloy AA2024-T3 is widely used in the aerospace industries.1–3) Due to the addition of alloying elements, the AA2024-T3 has exceptional mechanical properties.4–7) However, the presence of intermetallic compound particles in the aluminum alloy will form a local electrochemical cell with the aluminum alloy substrate, and the susceptibility of localized corrosion increases with the addition of alloying elements. The corrosion of aluminum alloys in aqueous environments has been extensively studied and found that the dissolved oxygen and the concentration of Cl− are important factors.8–10) The passive film is destroyed by Cl−, causing the aluminum alloy to corrode. Particles of intermetallic compounds observed in AA2024-T3 mainly possess Al2CuMg, corresponding to S-phase precipitate. The Mg and Al in the S-phase will preferentially dissolve when it is exposed to corrosive Cl− solutions causing Cu enrichment,11–13) and the localized corrosion rate will be accelerated by the residual Cu. The destruction rate of the passive film increases with an increase in the concentration of Cl− in the solution.14–16)
The common methods to protect the aluminum alloy from corrosion are using coating17,18) and inhibitors.19,20) However, these approaches have some disadvantages such as high cost and harm to the environment. It is a desire to develop an environmentally friendly, non-toxic, low-cost, and simple-to-handle corrosion inhibitor. Many researches have shown that metal cations can inhibit the corrosion of metals in aqueous solutions.21–27) Khedr et al.28,29) observed that transition and heavy metal cations might inhibit the corrosion of aluminum by trapping the electrons of cathodic reactions at low concentrations in neutral and acidic Cl− containing environments. Kato et al.30) reported that trace amounts of Al3+ present in 0.14 mM NaCl solution inhibit corrosion of carbon steel by forming an Al (OH)3·xH2O protective layer. Collazo et al.31) reported that Mg2+ reduces the dissolution rate of S-phase particles and inhibits the subsequent Al matrix corrosion. Islam et al.32) found that when the concentration of Zn2+ in NaCl solutions increases, the corrosion rate of mild steel in the solution decreases. From previous research, the effect of metal cations on corrosion behavior of AA2024 in Cl− contained aqueous solution must be considered.
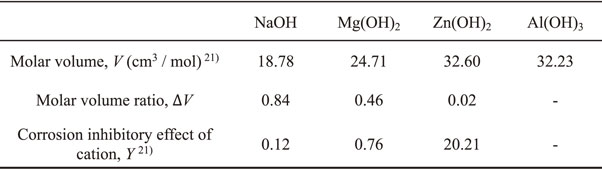
To better understand the role of metal cations in the corrosion of steel, Islam et al.25,27,33) used the hardness of metal cations based on the hard and soft acid and base (HSAB) concept. They found that the harder metal cations such as Zn2+ and Al3+ may incorporate with OH− existed on steel and then form a metal cation layer to protect the steel matrix from Cl− attack. However, Otani et al.21–23) concluded that the hardness of metal cations in the model freshwater could not fully explain the corrosion rate of mild steel. To explain the varying degrees of protection of metal cations, they established a new indicator, the corrosion inhibitory effect, Y, by combining the hardness with the molar volume ratio between the hydroxides of cations and γ-Fe2O3.21) The Y of the various metal cations are defined by eq. (1):21)
| \begin{equation} Y = X\times \Delta V^{-1}/10 \end{equation} | (1) |
| \begin{equation} \Delta V = |V_{\text{cat}} - V_{\text{sub}}|/V_{\text{sub}} \end{equation} | (2) |

The corrosion behavior of AA2024-T3 was investigated in different model freshwater. Immersion experiments and electrochemical measurements were used to determine the effect of metal cations. To better understand the relationship between corrosion behavior and the microstructure of AA2024-T3, a detailed microstructure analysis is performed. A mechanism for the corrosion inhibition of metal cations was proposed.
The specimens were cut from sheets of AA2024-T3 with 7 × 7 × 1 mm. For electrochemical testing, a conductive wire was attached to the specimens, and then the specimens were embedded in epoxy resin (Struers Ltd., Epofix Resin), leaving one side exposed for solutions. The specimens of all experiments were ground with SiC abrasive paper from #240 to #4000. After grounding, the specimens of immersion experiments were removed from the resin and immersed in the test solution. Before the test, the specimens were cleaned in ethanol and then in distilled water using an ultrasonic bath.
2.2 SolutionsThe following three model freshwaters were used as test solutions: 10 mM NaCl (Na–S), 0.5 mM MgCl2 (Mg–S), and 0.5 mM ZnCl2 (Zn–S), and the concentration of Cl− was adjusted to 10 mM with NaCl. The water used to prepare the model freshwater was highly purified: distilled twice and then refined in a water purifier (MILIPORE, Simplicity UV). The special analytical grade chemicals used in this investigation was purchased from Kanto Chemical Co. Ltd.
2.3 Immersion experimentsThe specimens were carried out in different model freshwater for 7, 14 and 21 d at 298 K under naturally aerated conditions. Before and after the immersion experiments, the mass change of the specimen was measured using a microbalance. The corrosion rate of specimens was calculated from the mass loss results during the immersion experiments by the eq. (3):
| \begin{equation} \text{Corrosion rate ($\unicode{x00B5}$m/y)} = \frac{\text{M}_{1} - \text{M}_{\text{2}}}{\text{D}\times \text{S} \times \text{t}}\times 3650 \end{equation} | (3) |
M1 and M2 are the mass of specimens before and after the immersion experiment in mg, D is the density of the specimen in g/cm3, S is the specimen exposed area in cm2 and t is the experiments duration in days.
The appearance of the specimens before and after the immersion experiments was examined by a digital camera (Canon Inc., 70D). The specimens surface and cross-section were observed using an SEM equipped with an EDS (JEOL Ltd., JSL6510-LA). The surface was analyzed using an XPS (JEOL Ltd., JPS-9200) with Al K as the X-ray source (1486.6 eV). The specimens cross-sections were prepared using a cross-sectional polisher (CP, JEOL Ltd., SM-09010). The cross-section of the specimen was analyzed by SEM and AES (JEOL Ltd., JAMP-9500F). Before each immersion experiment and observation, the specimens were cleaned ultrasonically with ethanol, then in distilled water and finally dried.
2.4 Electrochemical measurementsElectrochemical experiments were carried out in model freshwater and using a standard three-electrode cell with a potentiostat (IVIUM TECHNOLOGIES, Compactstat). Exposed surface area of the specimens used in electrochemical measurements was 0.49 cm2. Before the measurements, the specimens were immersed in the solutions for 3.6 ks (1 h) at 298 K to measure open circuit potential (OCP). Polarization measurements were taken to evaluate corrosion resistance in three model freshwaters and conducted from OCP to the cathodic and anodic directions separately at a scanning rate of 1 mV/s. Electrochemical Impedance Spectroscopy (EIS) measurements were performed at OCP with a modulation amplitude of 10 mV in the frequency range of 104 Hz to 10−2 Hz. EIS results were fitted by IVIUM software. Reproducible results were obtained throughout the electrochemical experiments.
Figure 1 shows the appearance of specimens after immersion experiments for 7, 14, and 21 d at 298 K. After immersion for 7 d, the Zn–S is covered by white and grey corrosion products. The specimens immersed in Na–S and Mg–S for 7 d are covered by silver grey corrosion products and it can also observe some black corrosion products on Na–S. As the immersion time, the Na–S and Mg–S are gradually covered by black corrosion products and fully covered after 21 d immersion. However, surface of the specimen immersed in Zn–S is partially covered by black corrosion products.

The appearance of specimens after immersed in the solutions for 7–21 d at 298 K.
The mass changes as a function of immersion time are shown in Fig. 2. There is no significant difference among the three solutions at short-time immersion (7 d). The mass changes of Na–S and Mg–S increased with immersion time, while the corrosion rate of Zn–S decreased with immersion time. The result suggests that the corrosion inhibition efficiency of specimens immersed in Zn–S is increased with time. Figure 2(b) shows the corrosion rate of specimens after 21 d immersion as a function of Y. In comparison to the other solutions used in this study, the results show that Zn2+ has the best corrosion inhibition ability for 2024 aluminum alloys. The correlation coefficient of determination of 0.93, showing that the corrosion rate is proportional to Y.

(a) Mass changes as a function of immersion time, and (b) corrosion rate after immersed for 21 d as a function of corrosion inhibitory effect of cation, Y.
Potentiodynamic polarization curves of the specimens in three different model freshwaters after 1 h immersion at 298 K is shown in Fig. 3. Zn–S shows higher cathodic current density than other solutions between −0.55 V and −1.0 V, which could be explained by the reduction of Zn2+. An oxygen diffusion limiting current in the cathodic branch is observed, which indicates that a mass-transfer step controls the cathodic reaction.36) From the anodic polarization results, the anodic current densities increase rapidly at potential above OCP, and passive region is not observed. The anodic polarization curves show no significant difference among three solutions.
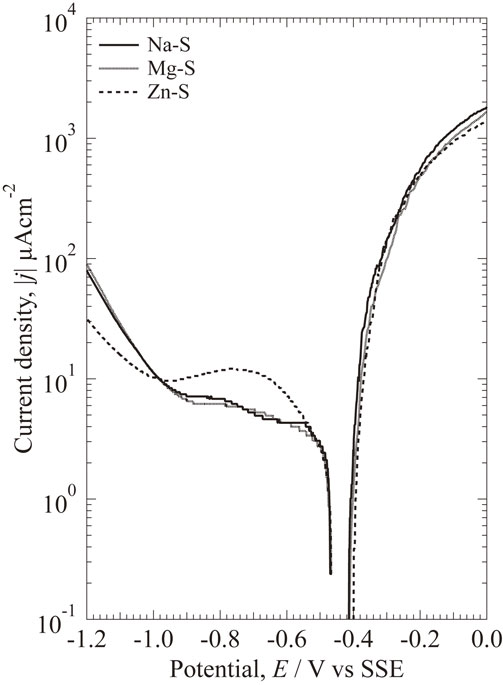
Potentiodynamic polarization curves of specimen after immersion for 1 h at 298 K.
EIS measurement was carried out to elucidate the influences of metal cations on corrosion of AA2024-T3 and estimated the interface structure between AA2024-T3 and model freshwater at the initial. Typical Bode plots of the specimens after 1 h of exposure to different model freshwater are shown in Fig. 4. From Fig. 4(a), the order of impedance |Z| at low frequencies is Zn–S > Mg–S > Na–S, indicating that Zn–S has the best corrosion inhibition ability among Na–S and Mg–S. This result is confirmed by the phase shift diagram (Fig. 4(b)). The phase shift indicates that Zn–S has the larger phase shift, implying that the protective film formed in Zn–S is better than those formed in the other model freshwaters. The equivalent circuit shown in Fig. 4(c) is proposed for simulating the protective film on specimens with a defect.24) Where Rsol, Rd, and Rct represent the resistances to model freshwater, protective film on the surface, and charge transfer, respectively. Qdl and Qf are constant phase elements corresponding to the electrical double layer in the defect and on the working electrode, respectively. The fitted lines are shown in the Bode diagram, which fitted well with the experimental plots.
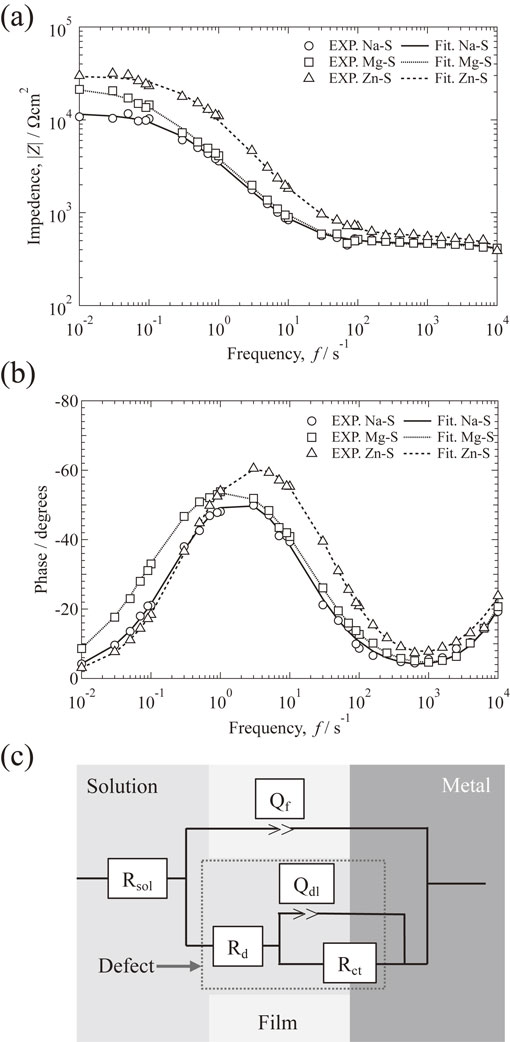
EIS results of specimen after immersion in the solutions for 1 h, Bode diagram of (a) impedance and (b) phase shift plots, and (c) equivalent circuit used to fit the EIS data at 298 K.
Table 3 shows the calculated electrochemical impedance parameters obtained from EIS measurements in different model freshwaters. Zn–S has the highest Rct among other solutions. This result means that the metal cations with large Y formed a metal cation layer, which may inhibit charge transfer (corrosion) reaction and permeation of Cl− into the films. The Qdl of Zn–S is the lowest among the solutions, indicating that Zn2+ may reduce the number of defects in the passive film of AA2024-T3 immersed in Zn–S.
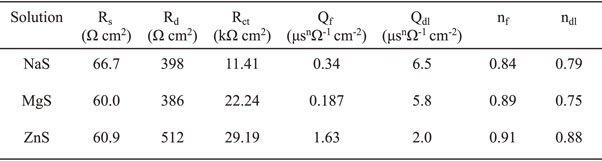
The immersion experiment results indicate that the appearance of specimens is changed with metal cations. Surface morphologies of specimens immersed in different model freshwaters for 7, 14, and 21 d at 298 K are shown in Fig. 5. After immersed for 7 d, coarse corrosion products are detected on the surfaces of Na–S and Mg–S, while no coarse corrosion products are detected on Zn–S. After 21 d of immersion, both Na–S and Mg–S are fully covered by large corrosion products, while Zn–S has no significant change during immersion.
Surface morphologies of specimen after immersed in different model freshwater for 7, 14, and 21 d at 298 K.
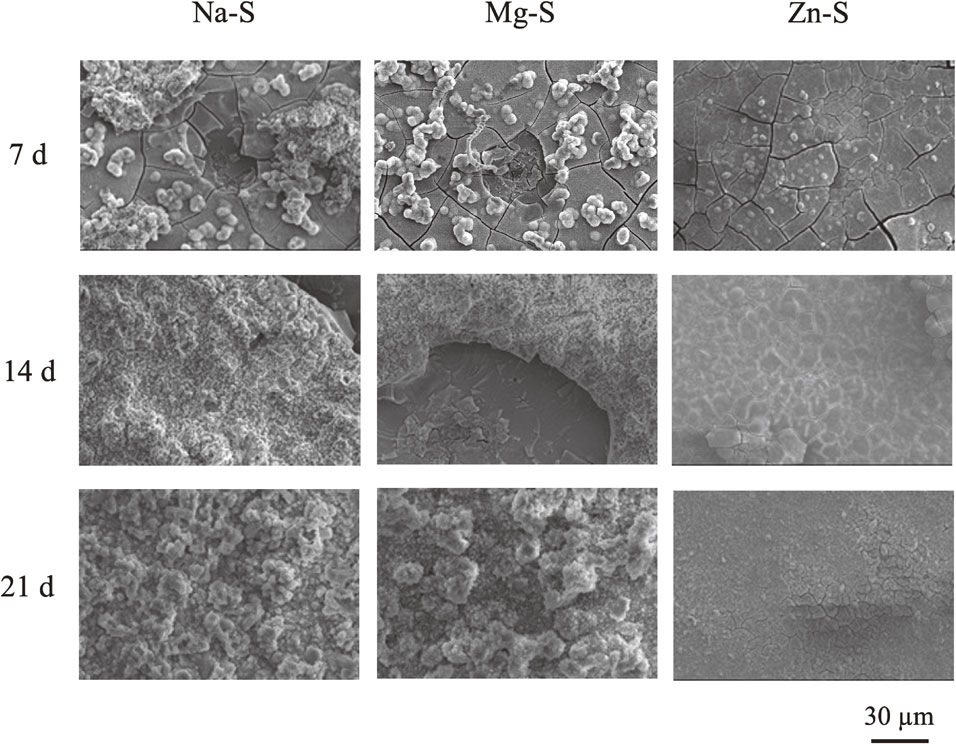
After the immersion experiments, the specimen surfaces were analyzed by XPS for a better understanding of the influence of metal cations on the oxide layer. Figure 6(a) shows the XPS wide spectra of specimens after immersion for 7 d in different model freshwaters. There are peaks of Zn 2p1/2 and Zn 2p3/2 after being immersed in the Zn–S. The presence of Na 1s or Mg 2p was not detected from specimens immersed in Na–S and Mg–S. The results suggested the presence of Zn compound on the specimens immersed in Zn–S. To investigate the chemical state of formed compound on AA2024-T3, the XPS narrow spectra of O 1s, Al 2p3/2, and Zn 2p3/2 in Zn–S were investigated and analyzed (Fig. 6(b)). The spectrum of O 1s may be deconvoluted into 530.5 eV and 532.0 eV, corresponding to the metal oxides and hydroxides.37) The spectrum of Al 2p3/2 may be deconvoluted into Al bond for Al2O3 (73.7 eV) and Al–OH (74.6 eV).38,39) The spectra of Zn 2p3/2 may be separated into two peaks (1021.6 eV and 1022.7 eV), and the peak at 1022.7 eV is attributed to Zn–OH and the peak at 1021.6 eV being Zn–O.37,40) These results indicate that the aluminum alloy may be protected by the Zn2+ contained corrosion products layer from Cl− attack and the layer inhibit the corrosion of Al matrix.
XPS (a) wide spectra of specimen surface after immersed in different model freshwater for 7 d, and (b) narrow spectra of O 1s, Al 2p3/2, and Zn 2p3/2.
Figure 7 shows the cross-sectional morphologies of the specimens immersed in (a) Na–S, (b) Mg–S, (c) Zn–S for 21 d at 298 K, and (d) EDS results of specimen immersed in Zn–S. The results show that specimens immersed in Na–S formed 10 to 20 µm thick corrosion product layer is observed, and the corrosion products and AA2024-T3 interface are rougher than in other solutions. The thickness of the corrosion products layer immersed in Mg–S is thinner than that of Na–S, and there is a large pit around the intermetallic compound particle. The specimen immersed in Zn–S shows the thinnest and smoothest corrosion product layer among the other solutions. In Fig. 7(d), a Zn-rich layer of corrosion products exists between the upper resin and the lower substrate. However, the central area marked as a dotted rectangle is clearly less bright than the other areas, which suggests that this area contained few Zn. Comparing the cross-sectional SEM images of Fig. 7(c), the corrosion is more serious in the dotted rectangle areas. In addition, the pitting corrosion in Zn–S is less serious than in Na–S and Mg–S, suggesting that Zn2+ were absorbed by passive film on Al matrix and formed protective metal cation layer. As a result, the passive film on Al matrix is significantly protected from the initial Cl− attacking and retard the corrosion rate of Al matrix.
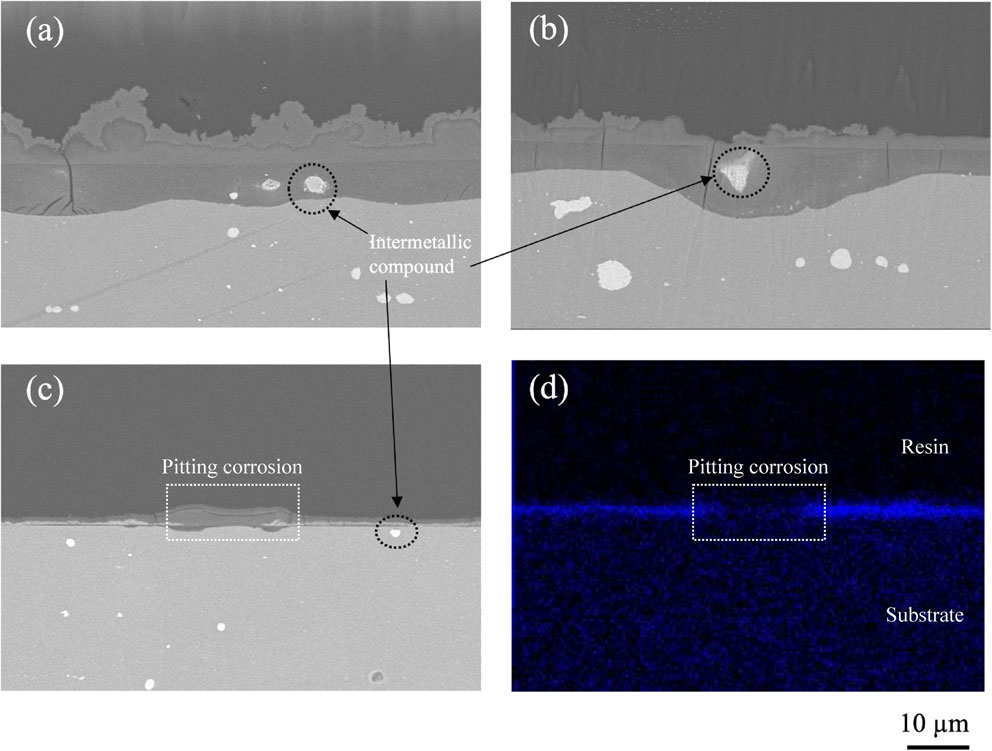
Cross-sectional morphologies of the specimens after immersed for 21 d at 298 K in (a) Na–S, (b) Mg–S, (c) Zn–S, and (d) EDS elemental mapping of Zn obtained from specimen after immersed for 21 d in Zn–S.
The cross-section of the specimen is further analyzed by AES to identify the position of Zn in the corrosion product layer formed on the AA2024-T3. Figure 8(a) shows SEM images of analyzed area of specimen cross-section, and Fig. 8(b) shows the AES point analysis results of specimen at selected points shown in the SEM image. Peaks of O and Al are shown in all analysis points, suggesting that Al oxide or hydroxides are formed. Zn peaks are only detected in the middle (+3 and +4) of the corrosion product layer. This result suggests that corrosion products (Al-rich layer/Zn-rich layer/Al-rich layer) with multilayered structure are formed.

(a) Cross-sectional morphologies of a specimen immersed in Zn–S for 21 d at 298 K, and (b) AES point analysis of corrosion products.
The results shown in the above sections suggest that Zn2+ exhibited the best corrosion inhibition effect among the used metal cations. A possible corrosion inhibition mechanism of AA2024-T3 immersed in model freshwater with different metal cations will be presented below.
The corrosion mechanism of AA2024-T3 in the solutions containing metal cations with small Y such as Na+ and Mg2+ is shown in Fig. 9(a) and (c). From the results of XPS shown in Fig. 6, Na+ may not form any compound and bond with the oxidate film on aluminum alloys. Alwitt41) reported that the passive film on aluminum alloys has a multilayer structure, outer hydroxide, and inner oxide, in model freshwater. The Cl− with high electronegativity easily reaches to passive film/Al matrix interface, destroying the passive films and causing the Al matrix to dissolve.40) Based on the HSAB concept, Mg2+ is classified as hard acid, and OH− is classified as a hard base. It is anticipated that Mg2+ and OH− will form a metal cation layer on the surface. However, the molar volume ratio between Mg (OH)2 and Al (OH)3 is too large to form a stable metal cation layer. Therefore, the Mg2+ cannot inhibit Al matrix from Cl− attacking.
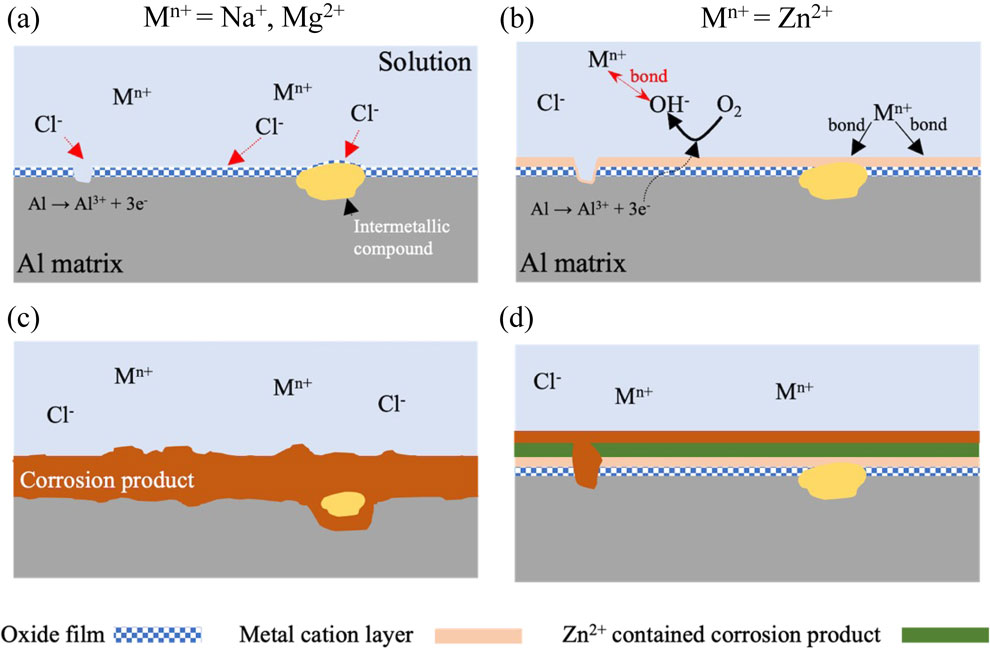
Corrosion mechanism of AA2024-T3 in model freshwater containing metal cations with (a), (c) small Y, and (b), (d) large Y.
The corrosion mechanism of aluminum alloys in the solutions containing metal cations with large Y such as Zn2+ is shown in Fig. 9(b) and (d). The potential difference occurs during the second phase and element segregation in aluminum alloys, resulting in a local electrochemical reaction of anodic metal oxidation at the defects (eq. (4)) and cathodic oxygen reduction (eq. (5)):
| \begin{equation} \text{Al} = \text{Al$^{3+}$} + \text{3e$^{-}$} \end{equation} | (4) |
| \begin{equation} \text{1/2O$_{2}$} + \text{H$_{\text{2}}$O} + \text{2e$^{-}$} = \text{2OH$^{-}$} \end{equation} | (5) |
The metal cations with large Y may easily be absorbed on the passive films and incorporated with OH− to form a metal cation layer on the AA2024-T3. From the immersion experiments, the corrosion rate of specimen immersed in Zn–S is the same as Na–S and Mg–S after short-time immersion. Zn did not observe near the interface between corrosion products and Al matrix by AES analysis. These results suggest that Zn2+ may not form any compound to protect Al matrix from Cl− attack at the early stage of corrosion. However, Zn peaks were detected in the middle of corrosion product as shown in Fig. 8. From the Pourbaix diagram,42) Zn2+ can precipitate as hydroxides at pH around 6. The solution pH may increase by oxygen reduction (cathodic, eq. (5)) reaction during corrosion of aluminum, when pH changes to higher than 6, and ZnO or Zn (OH)2 may precipitate on aluminum alloy. These results suggest that a dense metal cations layer inhibits Cl− attack and the Al matrix dissolution.
The influence of metal cations on corrosion behavior of AA2024-T3 in 10 mM Cl− model freshwater was investigated by surface analysis and electrochemical measurements. Following conclusions can be drawn.
This study was supported by The Light Metal Educational Foundation, Inc., and was conducted at Laboratory of XPS analysis, Hokkaido University, supported by the “Nanotechnology Platform” Program of the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.