2023 Volume 64 Issue 2 Pages 392-397
2023 Volume 64 Issue 2 Pages 392-397
To reduce CO2 emission, recycling Al scrap into wrought alloy is desired. However, impurity elements are inevitably contained in the Al scrap recovered from the society, and most of them are difficult to be removed by the current pyro-metallurgical processes. Mn is one of the major alloying elements of Al alloys and steels and is contained in Al scraps. Therefore, a removal method of Mn from Al is necessary. Mn easily forms an inter-metallic compound with Al; on the other hand, it is immiscible with Mg in the liquid state, which suggests that the repulsive interaction between Mg and Mn in molten Al enhances the precipitation of intermetallic compounds such as Al6Mn. This study proposed Mn removal from molten Al–Mg alloy through precipitation of Al6Mn inter-metallic compound. Molten Al–Mg alloy was equilibrated with Al6Mn, and the reducing limit of Mn concentration was thermodynamically discussed. Mn concentration becomes lower at higher Mg content of molten Al and lower temperature. The activity coefficient of Mn in molten Al–Mg alloy was increased with the addition of Mg, and the following equation was obtained as a function of temperature and molar fraction of Mg:

It was found that Al6Mn will precipitate due to the repulsive interaction of Mn and Mg when the Mg content of Al is increased. The thermodynamic analysis showed the possibility of reducing the Mn content of Al to 0.0030 mass% at 733 K and 34 mass%Mg by the present removal process.

Fig. 6 Contour lines of Mn content of molten Al–Mg alloy equilibrated with Al6Mn.
Recycling Al scrap is essential to achieve carbon neutrality because the amount of CO2 emission can be decreased to 3% of the production process of Al from natural resources.1–3) However, impurity elements are inevitably contained in the Al scraps recovered from society. Most of them are difficult to be removed by such current pyro-metallurgical processes as oxidation, gasification, and chlorination.4–6) Because the tolerance level of impurity contents of wrought Al alloys is much lower than cast alloys, Al scrap is mainly recycled into cast Al alloys, which are used for engines and transmissions of automobiles.2,7,8)
The electric car has been developed and will become more popular in the future. Therefore, the demand for automobile engines and transmissions will decrease, leading to a surplus of Al scraps.9) Accordingly, recycling Al scrap into wrought alloys is expected.3) Mn is one of the major alloying elements of Al alloys and steels. Therefore, Mn is often contained in the recovered Al scraps, and a removal method of Mn from Al is needed. Regarding the purification of Al, electro slag refining,10) the segregation process,11) and the precipitation method12–14) have been studied. The precipitation method is a molten alloy purification process through the precipitation of intermetallic compounds. However, Otaki et al.12) reported that the precipitation did not occur when the concentration of impurity elements was low. Considering the repulsive interaction between Fe and Mg, Shinomiya et al.15) proposed the removal method of Fe through the precipitation of Al3Fe intermetallic compound by adding Mg in molten Al, which contains Fe. Sekii et al.16) have also reported the removal method of Si in molten Al through the precipitation of Mg2Si intermetallic compound by adding Mg. Mn easily forms an intermetallic compound with Al; on the other hand, it is immiscible with Mg in the liquid state, which suggests that the repulsive interaction between Mg and Mn in molten Al enhances the precipitation of intermetallic compounds. Mg is an essential element in 5000-series aluminum alloys, and Al–Mg alloys with a higher Mg content due to Mn removal may be used as an Mg source for producing such Al–Mg alloys. Mg in Al is also an element that can be removed by gasification, oxidation or chlorination,4,6) if necessary. Accordingly, in this study, Mn removal from molten Al–Mg alloy through the intermetallic compound precipitation was proposed. According to the phase diagram of the Al–Mn binary system,17) Al6Mn coexists with the liquid phase at the melting point of Al. Therefore, molten Al–Mg alloy was equilibrated with Al6Mn, and the solubility of Mn was investigated. From the experimental results, thermodynamic data of the formation of Al6Mn inter-metallic compounds were derived. The reduction limit of Mn concentration was analyzed using the derived thermodynamic data.
Al2O3 crucible (o.d.: 39 mm, i.d.: 35 mm, height: 37 mm) containing 30 g of Al (99.99%) and 10 g of Mn (99.9%) was put in a high-frequency induction furnace. The samples were heated to 1300 K in an Ar at a flow rate of 300 cm3/min(s.t.p.) and held for 10 min to be melted. Then, the heating was stopped, the sample was cooled in the furnace, and Al–Mn alloy was obtained.
The Al2O3 crucible containing Al and Mg (99.9%) was put in the high-frequency induction furnace. The samples were heated to 873 K in 50 vol%H2–50 vol%Ar gas at a flow rate of 400 cm3/min(s.t.p.) and held for 10 min to be melted. Then, the heating was stopped, the sample was cooled in the furnace, and Al–Mg alloy was obtained.
The equilibrium experiments were conducted with an electric resistance furnace, shown in Fig. 1. A mullite tube sealed with silicone rubbers was vertically positioned at the center. Ar gas was introduced through an Al2O3 tube attached to the silicone rubber. The furnace temperature was measured with Pt–30Rh – Pt–6%Rh thermocouple and controlled with a P.I.D. controller. The experimental conditions are shown in Table 1. Al2O3 crucible (o.d.: 29 mm, i.d.: 24 mm, height: 51 mm) containing Al–Mn and Al–Mg alloys prepared preliminary was put in the mullite tube. The samples were heated to 823 or 873 K at 10 K/min and held for 19 h in Ar at a flow rate of 200 cm3/min(s.t.p.). Then, the crucible was withdrawn from the furnace and cooled in the air. The sample after the experiment was cut and taken for chemical analysis. Mg and Mn contents of the Al–Mg alloy phase were analyzed by an inductively coupled plasma–atomic emission spectrometry (ICP–AES). The compound phase of the sample was investigated by the X-ray diffraction method (XRD).

Schematic of Experimental apparatus and arrangement of samples.

Experimental results are summarized in Table 2, and the relationship between Mn and Mg content of molten Al–Mg alloy is shown in Fig. 2, together with their initial concentrations. The compound phase of the samples (No. 1, 3 and 6) was investigated by XRD and was identified to be Al6Mn. Mn content of the alloys increased from zero due to the dissolution of the Al–Mn alloy. The Mn concentration becomes lower at higher Mg content of the alloy and lower temperature, which indicates that the Mn content of molten Al can be decreased with increasing Mg content and lowering the temperature. The maximum value of the Mn concentration was 1.79 mass% at 873 K and 12.8 mass%Mg, and the minimum value was 0.07 mass% at 823 K and 38.2 mass%Mg.


Relationship between Mn and Mg contents of molten Al alloy.
The formation of the Al6Mn intermetallic compound was thermodynamically discussed. The equilibrium of molten Al–Mg alloy and Al6Mn(s) is expressed as eq. (1).
| \begin{align} &\text{6Al($l$, in Al–Mg alloy)} + \text{Mn($l$, in Al–Mg alloy)}\\ &\quad = \text{Al$_{6}$Mn(s)} \end{align} | (1) |
| \begin{equation} \Delta G_{(1)}^{\text{o}} = -189344 + 116.33T\quad (\text{J/mol}) \end{equation} | (2) |
| \begin{equation} \ln K_{(1)} = -\frac{\Delta G_{(1)}^{\text{o}}}{RT} = \ln \left(\frac{a_{\text{Al${_{6}}$Mn}}}{a_{\text{Al}}{}^{6}\cdot a_{\text{Mn}}} \right), \end{equation} | (3) |
| \begin{equation} \ln \gamma_{\text{Mn}}^{\text{o}} = -6\ln a_{\text{Al}} - \ln x_{\text{Mn}} - \ln K_{(1)} \end{equation} | (4) |
| \begin{equation} \ln \gamma_{\text{Al(${l}$,in Al–Mg alloy)}} = \alpha x_{\text{Mg}}{}^{2} \end{equation} | (5) |
| \begin{equation} a_{\text{Al}} = (1 - x_{\text{Mg}})\cdot \exp (\alpha x_{\text{Mg}}{}^{2}) \end{equation} | (6) |
| \begin{equation} \ln \gamma_{i} (T) = \frac{T_{0}}{T}\ln \gamma_{i} (T_{0}) \end{equation} | (7) |
| \begin{equation} \ln \gamma_{\text{Mn}}^{\text{o}} = -6\{\alpha x_{\text{Mg}}{}^{2} + \ln (1 - x_{\text{Mg}})\} - \ln x_{\text{Mn}} - \ln K_{(1)} \end{equation} | (8) |

Dependence of activity coefficient of Mn in molten Al–Mg alloy on Mg content at 823 and 873 K.
On the other hand, when Mn is diluted in Al–Mg–Mn ternary solution, the activity coefficient of Mn can be estimated by Toop’s equation expressed as eq. (9).20–22)
| \begin{align} \ln \gamma_{\text{Mn(${l}$,in Al–Mg alloy)}}^{\text{o}} & = \frac{x_{\text{Al}}}{x_{\text{Mg}} + x_{\text{Al}}} \cdot \ln \gamma_{\text{Mn(${l}$,in Al)}}^{\text{o}} \\ & \quad + \frac{x_{\text{Mg}}}{x_{\text{Mg}} + x_{\text{Al}}}\cdot \ln \gamma_{\text{Mn(${l}$,in Mg)}}^{\text{o}}\\ & \quad -(1 - x_{\text{Mn}})^{2} \cdot \frac{\varDelta G_{\text{Al–Mg}}^{Ex}}{RT} \end{align} | (9) |
| \begin{align} \ln \gamma_{\text{Mn(${l}$,in Al–Mg alloy)}}^{\text{o}} &= \alpha x_{\text{Mg}}{}^{2} + (\ln \gamma_{\text{Mn(${l}$,in Mg)}}^{\text{o}} \\ & \quad - \ln \gamma_{\text{Mn(${l}$,in Al)}}^{\text{o}} - \alpha)x_{\text{Mg}}\\ & \quad + \ln \gamma_{\text{Mn(${l}$,in Al)}}^{\text{o}} \end{align} | (10) |
| \begin{equation} \ln \gamma_{\text{Mn(${l}$,in Al–Mg alloy)}}^{\text{o}} - \alpha x_{\text{Mg}}{}^{2} = Ax_{\text{Mg}} + B \end{equation} | (11) |
| \begin{equation} \varDelta G_{i-\text{j}}^{Ex} = x_{i}x_{j}\sum_{\nu} L_{ij}^{\nu} (x_{i} - x_{j})^{\nu} \end{equation} | (12) |
| \begin{equation} \ln \gamma_{j}^{\text{o}} = \frac{L_{ij}^{0} + L_{ij}^{1}}{RT} \end{equation} | (13) |

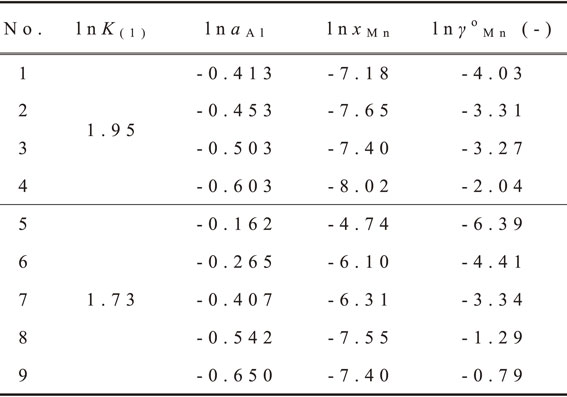
Determination of the activity coefficient of Mn in molten Al–Mg alloy at 873 K (the values of activity coefficient measured at 823 K were converted to those at 873 K using eq. (7)).
From eqs. (5) and (10), the activity coefficient of Mn in molten Al–Mg alloy was derived as a function of temperature and molar fraction of Mg as the following equation:
| \begin{align} &\ln \gamma_{\text{Mn(${l}$,in Al–Mg alloy)}}^{\text{o}} \\ & \quad = \frac{873}{T}(-0.459x_{\text{Mg}}{}^{2} + 17.94x_{\text{Mg}} - 8.80) \end{align} | (14) |
Using the thermodynamic data derived in Section 3.2, the reducing limit of Mn concentration in this method was analyzed. Equation (4) is rearranged as eq. (15).
| \begin{equation} \ln x_{\text{Mn}} = -6\ln a_{\text{Al}} - \ln \gamma_{\text{Mn}}^{\text{o}} - \ln K_{(1)} \end{equation} | (15) |

Solubility of manganese in molten Al–Mg alloy equilibrated with Al6Mn at 823 and 873 K.
As mentioned in Section 3.2, the degree of freedom is 1 under constant temperature and pressure. Therefore, the equilibrium Mg content and temperature can be determined when the solubility of Mn is fixed. Equation (15) is solved numerically, and the temperature and Mg content of Al alloy at fixed Mn contents were derived. The results are described on the phase diagram of the Al–Mg binary system in Fig. 6. In Fig. 6, the bold line shows the liquidus of Al, and the thin lines are the derived contour lines of fixed Mn contents. The experimental results are also plotted in Fig. 6. The numbers in the figure indicate the mass percentage of Mn of each contour line and experimental plot. It is found that the contour lines reasonably agree with the experimental results except for the results of 19.8 and 35.5 mass%Mg, 0.47 and 0.11 mass%Mn, at 873 K. The removal limit of Mn can be estimated from the results. As can be seen, Mn content can be decreased at higher Mg content and lower temperature. This is caused by the increase in the equilibrium constant because the reaction is exothermic and the increase in the activity coefficient of Mn due to the repulsive interaction between Mn and Mg. Another benefit of Mg addition is the decrease in the liquidus temperature. Thermodynamic analysis showed the possibility of reducing the Mn content of Al to 0.0030 mass% at 733 K and 34 mass%Mg by the present removal method. The addition of Mg is proved to remove Mn effectively through precipitation of intermetallic compounds containing Mn.

Contour lines of Mn content of molten Al–Mg alloy equilibrated with Al6Mn.
Al–Mg molten alloy was equilibrated with Al6Mn at 823 and 873 K, and the solubility of Mn was investigated. From the experimental results, thermodynamic data of the formation of Al6Mn inter-metallic compounds were derived. Using the derived thermodynamic data, the reducing limit of Mn concentration was analyzed, and conclusions are summarized as follows:
| \begin{equation*} \ln \gamma_{\text{Mn(${l}$,in Al)}}^{\text{o}} = -8.80,\quad \ln \gamma_{\text{Mn(${l}$,in Mg)}}^{\text{o}} = 8.67 \end{equation*} |
| \begin{align*} &\ln \gamma_{\text{Mn(${l}$,in Al–Mg alloy)}}^{\text{o}} \\ &\quad = \frac{873}{T}(-0.459x_{\text{Mg}}{}^{2} + 17.94x_{\text{Mg}} - 8.80) \end{align*} |