2023 Volume 64 Issue 7 Pages 1600-1604
2023 Volume 64 Issue 7 Pages 1600-1604
The temperature dependence of hardness in hypereutectoid steels with various microstructures was measured by using the small ball rebound hardness test, and the effect of carbon state on the high-temperature hardness was discussed. As-received and spheroidized cementite steels consisted of spheroidized cementite, graphite and ferrite matrix, and the volume fraction of graphite in the spheroidized cementite steel was larger than that in the as-received steel. Pearlite steel had ferrite and cementite lamellar structure without graphite. The characteristic hardening was detected above 700 K in all steels, suggesting that the solid solute carbon enhances the hardness even in hypereutectoid steels with high strength. The pearlite steel exhibited the highest hardness owing to the ferrite and cementite lamellar structure and no graphite. While the spheroidized cementite steel exhibited the lowest hardness, although the size of cementite was finer than that in the as-received steel. It was quantitatively demonstrated that the large volume fraction of graphite caused the low hardness in the spheroidized cementite steel.

Fig. 5 Coefficient of restitution as a function of temperature in as-received, pearlite and spheroidized cementite steels.
In some actual usage environment of structural materials such as turbines and boilers, the materials are exposed in high temperatures. Thus, the high-temperature mechanical properties are important. Until now, the various tests for measuring the high-temperature hardness or tensile properties have been conducted.1,2) However, these tests need specific equipment such as furnaces, indenters having sufficient hardness and chemical stability at high temperatures, and gas replacement devices to prevent the surface chemical reaction. Therefore, the hardness or tensile tests at a high temperature were more difficult compared to these tests at a room temperature.
Recently, a small ball rebound hardness test was developed as handheld device for rebound hardness test. This method calculates hardness from the ratio of the velocities of the impact ball after and before the impact on the specimen surface.3) The velocity of the small ball is very high in this hardness test, and the contact time between the impact ball and specimen is so short that heat cannot be transferred between them. Therefore, this hardness test is suitable for the simple high-temperature hardness test without specific equipment. Koga et al.4) measured the temperature dependence of hardness in an interstitial free (IF) steel, ultra-low carbon (ULC) steel, and eutectoid steel by using the small ball rebound hardness test. They revealed that the characteristic hardening occurred above 700 K in the ULC and eutectoid steels, whereas the hardness of IF steel continuously decreased with raising the temperature. Furthermore, by using in-situ neutron diffraction measurement during heating, it was demonstrated that the solid solution of ultra-low carbon (approximately 100 ppm) above 700 K led to the characteristic hardening of the steels.4) Thus, the presence of carbon is the key to the characteristic hardening in steels; however, it is not certain that the characteristic hardening also occurs in the hypereutectoid steel, which has a high carbon concentration and higher hardness than ULC and eutectoid steels. Additionally, the relationship between the high-temperature hardness and microstructure has not been deeply investigated, and the validity of measured high-temperature hardness has not been assured.
In this study, the temperature dependence of the hardness in hypereutectoid steels with various microstructures were measured by using the small ball rebound hardness test, and the effect of carbon state on the high-temperature hardness and the validity of measured hardness were discussed.
A commercial hypereutectoid steel (Fe–1.36%C–0.20%Si–0.24%Mn–0.01%P–0.02%S, in mass%) was used in this study. Three types of microstructures were fabricated by the heat-treatment, as shown in Fig. 1. As-received steel was a commercial hot-rolled specimen. The specimen after solution treated at 1273 K for 1.8 ks and air cooling was referred to as pearlite steel, and then the pearlite steel after heat-treatment at 1073 K for 86.4 ks and furnace cooling was referred to as spheroidized cementite steel in this study.

Heat-treatment diagram.
Figure 2 shows (a) schematic illustration and (b) whole image of the measurement stage for the small ball rebound hardness test at high temperatures. The specimen slid quickly from the furnace to the test position and then the small ball rebound hardness test (eNM3A10, Yamamoto Scientific Tool Laboratory Co., Ltd.) was immediately conducted, as shown in Fig. 2(a). The test was conducted five times at each temperature level from 400 K to 1173 K, and the specimen was reheated at the test temperature for 300 s in each test interval. The temperature when the test was conducted was measured from the thermocouple fixed in the hole on the side of the specimen (Fig. 2(b)). The coefficient of restitution representing the hardness in the rebound hardness test5) was calculated from the ratio of the velocities of the impact ball after and before the impact on the specimen surface. In the small ball rebound hardness test, an alumina ball with a diameter of 3 mm was used and the velocity of the ball was 10 m/s. The microstructure was observed by using an optical microscope (OM) and scanning electron microscopy (SEM). The specimens for these observations were finally polished by colloidal silica suspension (OP-S suspension, Struers Ltd.), and then etched by 3% nital. The chemical contents were measured by using energy dispersive X-ray spectroscopy.

(a) Schematic illustration and (b) whole image of measurement stage for small ball rebound hardness test at high temperature.
Figure 3 shows OM images in (a) as-received, (b) pearlite, and (c) spheroidized cementite steels. As-received steel consisted of coarse spheroidized cementites and ferrite matrix (Fig. 3(a)). Pearlite steel exhibited a completely different OM image from that in as-received and spheroidized cementite steels owing to its microstructure (Fig. 3(b)). The enlarged secondary electron image of the pearlite steel confirmed that the ferrite and cementite lamellar structure was formed in the entire of the steel (Fig. 4), although few proeutectoid cementites were present at prior austenite grain boundaries, as indicated by red arrows in Fig. 3(b). The spheroidized cementite steel consisted of relatively fine spheroidized cementites and ferrite matrix, similar to the as-received steel. However, the characteristic black regions increased in the spheroidized cementite steel from that in the as-received steel. Only carbon was detected from the line profile analysis of chemical contents along white line in the black region (Fig. 3(d)), indicating these black regions correspond to the graphites, which are more stable phase than cementite. Thus, the part of cementite transformed into graphite during the heat-treatment at 1023 K for a long time in the spheroidized cementite steel. Graphite area fraction was measured from the OM images and summarized in Table 1. The spheroidized cementite steel had ten times more graphite area fraction than that of the as-received steel. While the pearlite had no graphite. The lamella spacing of the pearlite steel was approximately 0.1 µm. It was relatively fine compared to general pearlite structures,6,7) suggesting that the cooling rate during air cooling was significantly fast because the lamella spacing strongly depends on the transformation temperature.7) The graphite in the as-received steel dissolved during solution treatment at 1273 K, and then the fast cooling gave no time to precipitate graphite once again, resulting in no graphite in the pearlite steel.

OM images for (a) as-received, (b) pearlite, (c) spheroidized cementite steels, and (d) line profile of chemical contents along the white line in the black region.
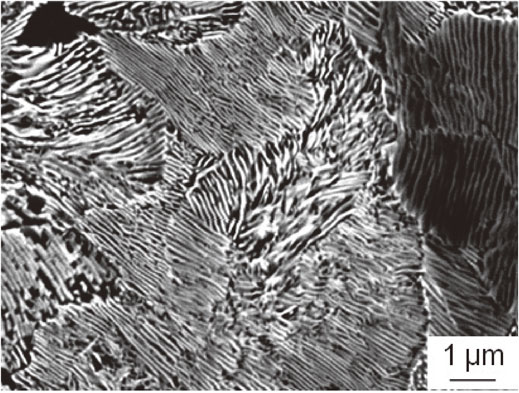
Secondary electron image of the pearlite steel.

Figure 5 shows the coefficient of restitution as a function of temperature in the as-received, pearlite, and spheroidized cementite steels. The coefficient of restitution continuously decreased until 700 K, indicating that the hardness decreased with raising the temperature. It is a general tendency of the temperature dependence of hardness in metal materials.8) Whereas, the coefficient of restitution increased above 700 K, corresponding to the characteristic hardening owing to the solution of carbon into ferrite matrix, as discussed previous study.4) Thus, it can be concluded that the characteristic hardening occurs even in the hypereutectoid steel which has significantly higher hardness at high temperature than that in the eutectoid steel.4) After the characteristic hardening, the coefficient of restitution decreased again, and the remarkable decrease of the coefficient of restitution at 1000 K in the as-received steel corresponded to the phase transformation from ferrite and cementite phases to austenite phase which is much softer phase than the cementite phase.

Coefficient of restitution as a function of temperature in as-received, pearlite and spheroidized cementite steels.
The pearlite steel had the highest coefficient of restitution among the steels. It was reported that the cementite spheroidization softens pearlite steels,9) thus, it can be reasonably understood that the high hardness of the pearlite steel is due to the lamellar structure of ferrite and cementite phases. The spheroidized cementite steel had lower hardness than the as-received steel, although the size of cementite was fine in the spheroidized steel and fine cementites should give high hardness according to precipitation strengthening mechanism.10) The area fraction of graphite was higher in the spheroidized cementite steel than that in the as-received steel. Thus, the graphite causes the low hardness in the spheroidized cementite steel and it was quantitatively discussed in the next section.
The precipitation of graphite occurs by following chemical formula:
| \begin{equation} \textit{Fe$_{3}$C} \to 3\textit{Fe} + C. \end{equation} | (1) |
Assuming that the initial volume fraction of cementite, i.e. no graphite, was 19.5%, which is estimated from the equilibrium diagram of Fe–C binary alloy and carbon concentration (1.36 mass%C), the volume fraction of each phase could be calculated from the volume fraction of graphite. In this analysis, the graphite area fraction (Table 1) was used for the calculation of the volume fraction of each phase. The densities in ferrite, cementite and graphite phases adopted 7.68 g/cm3, 7.87 g/cm3,11) and 2.26 g/cm3, respectively. The calculated volume fractions are summarized in Table 2. The increase of graphite leads to the decrease of cementite and the increase of ferrite. The volume fraction of cementite remarkably decreased in the spheroidized cementite steel caused by the large volume fraction of graphite, while the ferrite phase increased. Both ferrite and graphite are much softer phases than the cementite, thus, the large volume fraction of graphite led to the lowest hardness in the spheroidized cementite steel.

Based on the rule of mixture, the hardness of multi-phase materials can be calculated from the hardness (Vickers hardness: HV) and volume fraction (V) of each phase as follows:
| \begin{equation} \mathit{HV}_{\textit{all}} = \mathit{HV}_{a} \cdot V_{\alpha} + \mathit{HV}_{\textit{Fe${_{3}}$C}} \cdot V_{\textit{Fe${_{3}}$C}} + \mathit{HV}_{C} \cdot V_{C}. \end{equation} | (2) |
| \begin{equation} \mathit{HV} = 24\exp(4.0 \times eNM3A10) \end{equation} | (3) |

These results assure that the small ball rebound hardness test can easily measure the hardness at high temperature and the measured hardness was valid even at high temperature in the view of the microstructure. The state of carbon is important factor for the high temperature hardness because solution of carbon lead to significant improvement in the hardness but the precipitation of graphite caused the low hardness.
The temperature dependence of hardness in hypereutectoid steels with various microstructures was measured by using the small ball rebound hardness test, and the effect of carbon state on the high-temperature hardness was discussed.
Part of this work was financially supported by Iketani Science and Technology Foundation (0311099-A) and the Grant-in-Aid for Scientific Research (KAKENHI) Grant No. 20K14605. This work was also supported by JKA and its promotion funds from KEIRIN RACE.