2023 Volume 64 Issue 8 Pages 1879-1885
2023 Volume 64 Issue 8 Pages 1879-1885
In this paper we review the use of cold rolling for the enhancement of magnesium-based alloys. After a short description of the technique, we discuss the main systems that have been investigated in the recent years. Namely pure magnesium and it’s hydride, Mg–Pd, Mg–Al, Mg–Cu, Mg–Fe, and Mg–Ni.
Fig. 1 Schematic of cold rolling horizontal set-up (left) and vertical set-up (right) Reprinted from Ref. 29) with permission.
Hydrogen storage is a key component for the establishment of hydrogen as an energy vector. Presently, the main ways of storing hydrogen are in high-pressure tanks or in the liquid state. These techniques are currently used in some applications but they are not suitable for many potential usages. This is due to either the high pressure required for gaseous storage (up to 700 bars) or to the cryogenic temperature (20 K) needed for liquid hydrogen. Metal hydrides have a large range of temperature and pressure of operation. An important advantage of metal hydride is the high volumetric density which makes the hydrogen tank compact. Metal hydrides are promising candidates for many stationary and mobile hydrogen storage applications but presently there are only a few alloys that could meet the criteria of commercial applications.
Metal hydrides for hydrogen storage have been investigated for more than 50 years.1) There is a wide variety of metal hydrides systems, each one having its advantages and disadvantages.2–4) However, more research is still needed to find better metal hydrides that will meet all requirements of commercial applications such as high gravimetric capacity, temperature of operation near the room temperature, low cost, easy first hydrogenation, safety, etc. Because of its high gravimetric capacity (7.6 wt.%), magnesium and magnesium-based alloys are attractive for hydrogen storage.5–8)
In the development of new metal hydrides, ball milling has played an important role especially for the formation of new alloys, obtaining a nanostructured material and adding a catalyst.3,9) Despite the effectiveness of ball milling in the synthesis and/or improvement of metal hydrides hydrogen storage characteristics, this method may not be the most appropriate for large-scale synthesis. Recently, other mechanical deformation methods have been used for the development of metal hydrides. The main ones are: Equal Chanel Angular Pressing (ECAP),10–14) High-Pressure Torsion (HPT),15–22) Cold Rolling (CR),23–25) or a combination of them.14,26–28) As seen in these references, ECAP and HPT have been used much more than CR in the field of metal hydrides. However, it is our opinion that CR may be more suitable for large-scale production. Most of the investigation involving CR for metal hydrides has been on magnesium and magnesium-based alloys mainly because magnesium is ductile even though it has strong work hardening. Therefore, in this paper we will review the technique of cold rolling applied to magnesium-based metal hydrides. It should be stressed that comparison between different investigations is particularly difficult in the case of cold rolling technique. The efficiency of CR depends on many parameters such as rolling speed, thickness reduction after each rolling pass and nature of the rolled materials. As these parameters are not always reported in the literature the assessment between different studies is mainly qualitative. In any case, scaling up the technique will require to find the optimum conditions for each particular material and level of production.
In cold rolling, the material is processed by introducing it between rollers where it is compressed and squeezed. The term ‘cold rolling’ is used when the temperature of the metal is below its recrystallization temperature. When the rolling is done at a temperature higher than the recrystallization temperature, the term hot rolling should be used. In the field of metal hydrides, most processes are at room temperature thus, in this paper we will solely discuss the cold rolling (CR) technique.
Cold rolling is a technique designed to process flat plates and foil. However, metal hydrides are usually in powder form and horizontal rolling of powder is cumbersome. This problem could be easily solved by simply arranging the two roll side by side horizontally instead of vertically.29) Figure 1 shows the schematic of the horizontal and vertical set-ups.
Schematic of cold rolling horizontal set-up (left) and vertical set-up (right) Reprinted from Ref. 29) with permission.
The vertical arrangement makes the feeding and collecting of powder much easier. Although this arrangement works well on the laboratory scale, it may be harder to implement at industrial levels.
When rolling is done in a conventional way, i.e., on plates, the effect on the material could be formally analyzed. This has been done by Fleck et al.30,31) with a stronger algorithm proposed by Le and Sutcliffe.32) An important difference of cold rolling with most other SPD techniques is that the grain boundaries have low angle grain boundaries while most other techniques give materials with high angle boundaries.33) Also, contrary to HPT and ECAP processes in which the deformation is mainly shear, in cold rolling the deformation is mostly plane strain compression. This means that, the strains are approximately: ε11 = −ε33 and all other strains ε22 = ε12 = ε13 = ε23 = 0. Where the subscripts 1, 2 and 3 respectively refers to rolling direction (RD), transverse direction (TD) and normal direction (ND). The effective strain is then equals the von Mises equivalent strain.34)
| \begin{equation} \varepsilon = \sqrt{\frac{2}{3}} \varepsilon_{11} \end{equation} | (1) |
In the case of metal hydrides, as they are usually brittle, when cold rolling is performed, the most likely effect is just crushing and breaking down particles.
A few review papers give a broad description of the use of cold rolling on metal hydrides.13,36–38)
Cold rolling of pure magnesium is difficult because, even if magnesium is ductile, it has only four slipping planes.39) However, von Mises criterion requires that five independent slip systems must be present in order to have a uniform deformation without cracks at the grain boundaries.40) Therefore, a magnesium plate will become brittle as the number of rolling passes increases. This is not so dramatic for hydrogen storage purposes as defects could act as nucleation points for the formation of the hydride phase. However, it makes the rolling process difficult from a practical point of view.
Despite being quite difficult to cold roll, magnesium and magnesium-based alloys have been the main subject of investigations of the effect of cold rolling on hydrogen storage materials.
As it is in the powder form and is brittle, the hydride MgH2 was actually investigated as early as pure magnesium. Lang and Huot showed that cold rolling MgH2 five times in air gives hydrogenation and dehydrogenation kinetics almost as fast as ball milling 30 minutes in argon (see Fig. 2).41)

Normalized kinetic absorption at 623 K and under 2.0 MPa hydrogen pressure of MgH2 in as-received, cold rolled five times, and mechanically milled states. Reprinted from Ref. 41) with permission.
Leiva and Floriano showed that a combination of cold rolling and short-time ball milling may be better to get enhanced hydrogenation behaviour did cold rolling on MgH2.26,42–44) Floriano et al. also showed that cold rolling is a good technique to incorporate additives to MgH2.45) Cold rolling MgH2 with transition metal and their oxides was investigated by Vincent et al.46) and Bellemare and Huot.47) It was found that, for the pure elements, ball milling and cold rolling gave similar hydrogenation/dehydrogenation kinetics, with Ni, V and Nb being the most effective catalysts.46) The oxides NiO and Nb2O5 gave the fastest desorption kinetics but ball milling 30 minutes was more efficient than cold rolling 5 times in air.47) El-Eskandarany et al. prepared MgH2/Nb2O5 nanocomposite by first cold rolling magnesium 200 times followed by reactive ball milling under hydrogen for 100 hours, cold rolling the resulting powder 100 times and finishing by ball milling with addition of Nb2O5 for 50 hours.48) They found that the powders exhibited significant improvement in terms of kinetics and decomposition temperature. However, the extended preparation time and complexity may not be so suitable for industrial applications.
Cold rolling is usually performed in air but Márquez et al. investigated cold rolling of MgH2 under an inert atmosphere and also studied the effect of rolling speed.49) Rolling under inert atmosphere reduced contamination and they concluded that cold rolling under an inert atmosphere seems to be an excellent alternative for ball milling. The composite MgH2–LaNi5 was prepared by Marquez et al. using cold rolling under argon atmosphere.50) The composites had LaNi5 particle size lower than 10 µm and faster hydrogen absorption/desorption kinetics than pure MgH2.
El-Eskandarany et al. cold rolled up to 200 times MgH2 inside a stainless steel tube.51) The reduction after each rolling pass was not given but, as MgH2 is brittle, there is no issue of cold working. The 200 times rolled sample was then ball milled for 3 to 50 hours. They found a new metastable phase of fcc-MgH2 (Fm-3m(225)), with a lattice parameter of 0.4436 nm that could desorb at a temperature between 448 K and 523 K in a few minutes.
Jain et al. found that MgH2 could be used as an additive to pure magnesium in order to make the first hydrogenation faster.52) They first cold rolled pure magnesium in the air for 25 passes. The rolled samples were then ball milled for 30 minutes with the addition of 5 wt.% of MgH2. They found that, the addition of MgH2 greatly improved the first hydrogenation kinetics. This was explained by MgH2 acting as nucleation points for the formation of the hydride phase in pure magnesium. The rate-limiting step of hydrogenation/dehydrogenation of cold rolled MgH2 has been investigated by Lang et al.53) They found that the rate-limiting step for absorption is diffusion (3 dimension diffusion with decreasing interface velocity) while for absorption it is CV3D model (3 dimension growth with constant interface velocity). From NMR (Nuclear Magnetic Resonance) measurements, they showed that cold rolling MgH2 does not increase the rate of hydrogen diffusive hopping through the MgH2 lattice.
The resistance to hydrogen cycling of cold rolled MgH2 was investigated by Grill et al.54) They observed fast kinetics and high storage capacity for the first hydrogenation/dehydrogenation cycles (623 K, 10 bar absorption, 0.001 bar desorption) but it deteriorated with cycle number. After 100 cycles the capacity was reduced to 70% of its initial value. The degradation was explained by the limited diffusion of hydrogen within the hydride phase, and a gradual annealing leading to an insufficient number of nucleation sites.
It is well known that, upon rolling, the hexagonal structure of magnesium will develop a texture along (002).55) The correlation between hydrogen storage properties and texture has been investigated by Jorge et al.14) They concluded that higher capacities, faster kinetics and lower desorption temperatures correlate directly with the amount of (002) texture but that this effect tends to disappear with an increasing amount of texture. As the cold rolled magnesium is in the form of a plate, the specific surface is very small and negatively impact the hydrogenation kinetics. The established way is to file the plate in order to get small ‘chips’. The effect of such filing has been investigated by Lang et al.56) They found that filing cold rolled samples gives faster kinetics than filing the unprocessed magnesium plates.
Various commercial magnesium alloys have been investigated. Amira and Huot investigated AZ91D and three experimental creep resistant magnesium alloys (MRI153, AXJ530, ZAEX10430) in the as-cast and die-cast state.57) The alloys were first cold rolled in air 50 times. Thereafter the plates were ball milled 30 minutes with the addition of 5 wt.% of MgH2. All alloys, except AXJ530 showed better hydrogen storage properties after cold rolling than pure magnesium. Figure 3 shows the first hydrogenation kinetics of as-cast and die-cast AZ91D alloys.
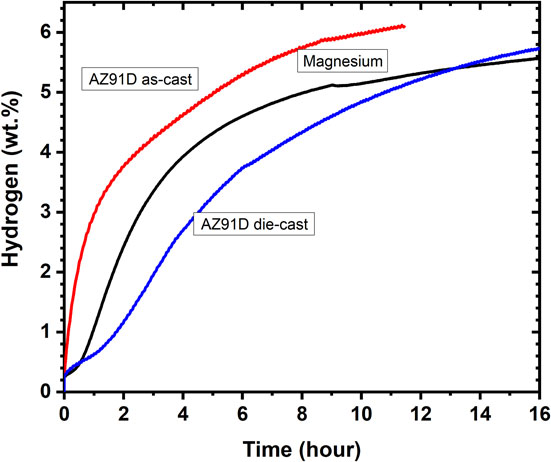
First hydrogenation kinetics at 623 K under 2.0 MPa of hydrogen pressure of pure magnesium, as-cast and die-cast AZ91D alloys. Adapted from Ref. 57) with permission.
Surprisingly, the as-cast alloys had better properties than dis-cast ones. It was explained by the more important phase segregation at the grain boundaries in the as-cast alloys.57,58) Leiva et al. compared the effect of HPT, cold forging (CF) and cold rolling (CR) on the first hydrogenation of commercial magnesium alloy and found that cold rolling gave a much faster hydrogenation kinetics.59,60) Soyama et al. investigated the addition of mischmetal to magnesium alloy ZK60 using various severe plastic deformation techniques and combination of them.61,62) They found that the best combination was ECAP followed by CR. They attributed the improvement to the fact that CR break down the intermetallic net of second phases, leading to a wide distribution of sizes, including in the nanometre range.62) Silva et al. added mischmetal to ZK60 and found excellent hydrogen storage properties after the cold rolled samples were filed.63) Jorge et al. also concluded that a combination of ECAP and CR is optimum to get fast kinetics for the alloys AZ91 and AM60D.64)
Floriano et al. studied the effect of rolling temperature on the alloy AZ91.65) Comparing rolling at room temperature and rolling samples that were previously immersed in liquid nitrogen, they found that rolling at low temperature produced samples that had faster hydrogen sorption/desorption kinetics and higher capacity compared to the room temperature ones. It was explained by the presence of microcracks and high areas of exposed interfaces in the sample produced at low temperature. Silva et al. investigated ZK60 alloy with mischmetal addition that was prepared by ECAP followed by CR at low temperature.66) The samples presented highly oriented grains along to the (002) plane with many cracks and voids. This resulted in an activation kinetics that was faster than the sample processed at room temperature. El-Eskandarany et al. recycled Mg-machining chips by first melting them and thereafter subjecting the ribbons to cold rolling and ball milling.67) Cold rolling produced small crystallites and enhanced hydrogenation/dehydrogenation kinetics. Donandey et al. also investigated different techniques (ball milling and cold rolling) on wastes of Mg-based alloy Elektron 21 (UNS-M12310).68) They found that the best kinetics are obtained when cold rolling is performed before ball milling. Dupim et al. have shown that for MgH2, TiH2, and ZrH2, cold rolling enhance the microwave-assisted desorption.69)
4.2 Mg–Pd alloysThe system Mg/Pd was first studied by Takeichi et al.70) They found that, after cold rolling and activation at 573 K, the intermetallic Mg6Pd is formed. Dufour and Huot compared the synthesis of Mg–Pd compounds by cold rolling and ball milling.23,71) They found that, for the Mg+2.5 wt.%Pd system, cold rolled samples have a microstructure where palladium is evenly distributed in magnesium. However, the palladium particle size was almost one order of magnitude bigger in the laminated compound compared to the ball milled. Nonetheless, the first hydrogenation (activation) of laminated sample was much faster than for the ball-milled sample. Figure 4 shows the first hydrogenation kinetics of Mg+2.5 wt.%Pd after cold rolling 30 times in the air. It is clear that cold rolling is more efficient than ball milling 2 hours in a Spex high energy mill.
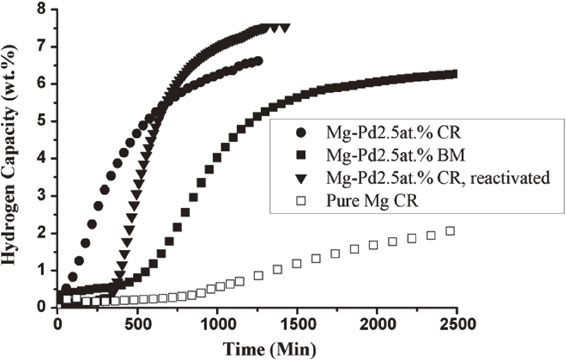
Activation curve of ball milled Mg–Pd 2.5 at%, cold rolled Mg–Pd 2.5 at%, and cold rolled pure magnesium. Activation temperature 623 K, hydrogen pressure 1.3 MPa. Adapted from Ref. 23) with permission.
After five hydrogenation/dehydrogenation, the cold rolled powder was stored in the air for one month. The powder was then cold rolled again five times. We see that the air exposed powder has an incubation time but thereafter the kinetic is quite fast and the absorption complete.
4.3 Mg–Al alloysThe system Mg–Al was investigated by Suganuma et al. who reported the formation of single phase Mg17Al12 by cold rolling followed by annealing at 673 K.72) However, upon hydrogenation, a disproportionation to MgH2 and Mg2Al3 occurred but a single phase Mg17Al12 was recovered after dehydrogenation.
4.4 Mg–Cu alloysThe system Mg/Cu have been studied by Takeichi et al.73) They found that the laminate presents a sub-micrometre structure with dislocations and stacking faults which enables the composite to reversibly absorbs hydrogen. Tanaka et al. prepared super-laminates Mg/Cu samples of different thickness layers by changing rolling reduction of each pass.74) They thus obtained ‘fine’ microstructure where Cu layers are 1 µm at most, ‘medium’ where the Cu thickness is about 2.5 µm, and ‘coarse’ that has Cu layers of 8 µm maximum thickness.74) They found that the ‘fine’ microstructure has the fastest kinetics but the maximum capacity reached was about 1.0 H/M while the ‘coarse’ sample had much slower kinetics but reached a higher capacity of H/M = 1.25. They explained this by the fact that, for the fine and medium samples the diffusion length between Mg and Cu is shorter than in the coarse sample and thus, the formation of MgCu2 will be privileged over the formation of MgH2.
4.5 Mg–Fe alloysLeiva et al. investigated the effect of adding a small amount of iron to magnesium by cold rolling.75) The best hydrogenation kinetics were for the samples to which iron was added in the form of continuously unwound wool. The ternary hydride Mg2FeH6 was prepared by Jung et al. by first cold rolling powder mixture of Mg and Fe.25) As Mg and Fe are totally immiscible, after rolling no intermetallic was formed. However, from Miedema’s rule of reversed stability a non-stable alloy will form a stable hydride.76) Therefore, it is expected that Mg2FeH6 could be formed. Jung et al. found that, with increasing numbers of rolling passes, the Mg and Fe layers become thinner and makes the formation of Mg2FeH6 faster.25)
4.6 Mg–Ni alloysTo our knowledge, one of the first investigations of cold rolling Mg and Ni was made by Ben Ameur and Yavari have shown that Mg/Ni multilayers could be amorphized by repetitive rolling even if the heat of mixing is slightly negative.77,78) The synthesis of a magnesium-based metal hydride alloy by cold rolling was made by Ueda et al. who tried to obtain Mg2Ni by cold rolling the raw elements followed by heat treatment at 673 K for 4 hours.79) They found that, for the stoichiometry 2Mg + Ni, a single phase Mg2Ni was obtained, and the sample could be completely hydrogenated to Mg2NiH4. The formation of Mg2Ni was explained by interdiffusion between Mg and Ni during heat treatment.79) Pednault et al. investigated the structural and electrochemical evolution of 2Mg–Ni cold-rolled samples as a function of the number of rolling passes as well as heat treatment.24,80) The best result was obtained by a succession of rolling, heat treatment and further rolling. Using this technique, they obtained a material having an initial discharge capacity of 205 mAh g−1, which is quite similar to that obtained with ball-milled Mg2Ni alloy.80) Leiva et al. studied the effect of cold rolling on a melt-spun alloy of composition Mg97Ni3.81) Cold rolling introduced a strong (002) texture in the Mg phase. The first hydrogenation of the melt-spun + cold rolled sample was faster than the melt spun alloy, the main difference being the almost total disappearance of incubation time for the cold-rolled sample. Révész et al. used a combination of ball milling and cold rolling or Equal Channel Angular Pressing (ECAP) on Mg2Ni alloy.82) They first synthesized Mg2Ni by ball milling Mg and Ni powders for 10 hours. The resulting powder was then subjected to cold rolling or ECAP. They found that cold rolling resulted in an important broadening of crystallites size distribution and that after 4 rolling passes the average crystallite size actually increases from 15 ± 3 nm for the ball milled powder to 34 ± 5 nm. Further rolling to 10 passes get the average crystallite size back to 18 ± 3 nm. Cold rolling decreases the hydrogen uptake capacity compared to the ball milled powder but increases the kinetics. El-Eskandarany et al. cold rolled magnesium rods and afterward spray coated them with Ni.83) In their paper they mention that 300 rolling passes were performed. This is very surprising as it is well known that rolling induce cold working in magnesium. But, as the reduction at each pass is not mentioned in their paper, it may be that the reduction at each pass was very small. After spraying with Ni, the Mg sheets showed good absorption/desorption kinetics. Huot and Tousignant directly cold rolled the Mg2Ni alloy.84) Rolling was performed on a Durston DRM 130 apparatus. The powder was rolled between stainless steel plates in order to prevent contamination from the rolls. Figure 5 shows the X-ray powder diffraction in as received and after 5, 12, and 25 rolling passes.

X-ray powder diffraction of Mg2Ni alloy in the as-received state and after cold rolling. Reprinted from Ref. 84) with permission.
Figure 5 clearly shows that cold rolling has for effect of broadening the Bragg’s peaks of the alloy. This indicates that the crystallite size decreases and that microstrain increases. The numerical values of crystallite size and microstrain are shown in Table 1. We see that there is an important reduction of crystallite size after only five cold rolling passes. Microstrain appears after 12 rolling passes.
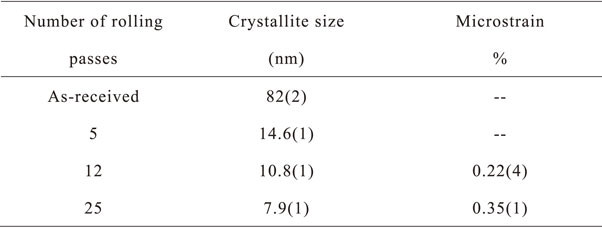
Figure 6 shows the first hydrogenation kinetics of as-received and cold rolled samples. Just five cold rolling made the hydrogenation kinetics much faster. Further rolling slightly increased the kinetics but we see that for 25 rolling passes the capacity is smaller. This may be due to the formation of oxide because all rollings were performed in the air.

First hydrogenation kinetics at 623 K and under 2.0 MPa of hydrogen of Mg2Ni alloy in the as-received and cold rolled states. Reprinted from Ref. 84) with permission.
The system Mg/Ti/Ni was investigated by Mori et al. and they found that the amount of Ni helped in the activation of the material.85) However, after hydrogenation only MgH2 could be dehydrided, TiH2 being too stable under the experimental conditions.
4.7 Other alloyA few more complex alloys containing magnesium have been investigated. Boidin et al. investigated the LaCuMg8 alloy which crystallizes in the La2Mg17 structure type.86) It was found that cold rolling on the LaCuMg8 synthesized by melting resulted in fast first hydrogenation.
Cold rolling technique has been shown to be an interesting technique to synthesize metal hydrides with enhanced hydrogen storage properties. The main benefits being a faster first hydrogenation, faster kinetics, nanocrystalline structure and formation of intermetallic under milder temperature and pressure conditions. One of the main drivers in using cold rolling is that this technique is potentially easier and cheaper to scale up compared to the other severe plastic deformation techniques such as high energy milling, ECAP and HPT. However, there is still a large field to explore before this technique could be used at a large scale. For example, the effect of the reduction ratio at each rolling pass, speed of rolling, and temperature have been little studied. Also, the rolling configuration (horizontal vs. vertical) and the required number of passes should be considered.