2024 Volume 65 Issue 1 Pages 27-36
2024 Volume 65 Issue 1 Pages 27-36
This study investigated the effects of potassium amyl xanthate (KAX) and oxidation treatment using hydrogen peroxide (H2O2) on the selective flotation of copper concentrates containing arsenic-bearing copper minerals. The mineralogical analysis revealed that enargite and chalcopyrite were the main arsenic-bearing copper and copper sulfide minerals, respectively, in the copper concentrate. KAX treatment at pH 9 improved the recoveries of copper sulfide and arsenic-bearing copper minerals. However, arsenic-bearing copper minerals floated more rapidly than copper sulfide minerals, indicating better separation selectivity. The separation selectivity of the KAX treatment was significantly improved at pH 10. H2O2 treatment was found to selectively improve the recovery of arsenic-bearing copper minerals. A combination treatment using 0.1 M H2O2 and 60 g/t of KAX at pH 9 enhanced the separation selectivity in the selective flotation of copper sulfide and arsenic-bearing copper minerals by producing a copper concentrate with the lowest arsenic grade and highest copper grade in tailings compared to those obtained from separated KAX and H2O2 treatments.

Arsenic is an undesirable element in a copper concentrate owing to its toxicity and harmful impact on human health and the environment, reducing the quality and economic value of the concentrate.1–6) Studies on minimizing the amount of arsenic by separating arsenic-bearing copper minerals from copper concentrates have attracted increasing attention. Flotation is a promising separation method used in these studies.7–16) Separation using flotation relies mainly on the difference in surface hydrophobicity between the targeted and undesirable minerals. This difference can be achieved by adding collectors, that is, chemicals that improve surface hydrophobicity, or depressants, that is, chemicals that alter surface hydrophobicity.17,18)
Previous studies have shown that selective flotation via oxidation treatment is a promising method for the selective separation of arsenic-bearing copper minerals (enargite and tennantite) and copper sulfide minerals (chalcopyrite, bornite, and chalcocite).8,9,11,12,14,15,19,20) However, these studies used a pure and binary mineral mixture to investigate the flotation behavior. The application of oxidation treatment for the flotation of complex copper ores and concentrates containing arsenic-bearing copper minerals has been reported.7,13) Lee et al.7) demonstrated that selective flotation of a copper concentrate containing enargite was feasible using sodium hypochlorite (NaOCl) as a depressant for copper sulfide minerals at pH 11.6. However, they did not investigate the effect of collectors on selective flotation behavior. Thayebi-Khorami et al.13) reported unsatisfactory separation of enargite and non-enargite copper minerals under oxidation conditions. They also suggested flotation under reducing conditions to selectively separate enargite and non-enargite copper minerals at pH 11.
A previous study19) indicated that selective flotation of a binary mixture of arsenic-bearing copper minerals and primary copper sulfide minerals was feasible with the addition of potassium amyl xanthate (KAX) as the collector. The separation selectivity can be enhanced via pre-oxidation treatment using a hydrogen peroxide aqueous solution to reduce the floatability of copper sulfide minerals, that is, chalcopyrite.19,21–23) However, the applicability of this method for a more complex copper–arsenic concentrate has not been reported in detail.
In this study, the effects of KAX addition and oxidation treatment using hydrogen peroxide on the selective flotation of copper concentrates containing arsenic-bearing copper minerals under various pH conditions were investigated. Furthermore, the optimum conditions for producing a “clean” copper concentrate with the lowest arsenic contamination and the highest grade of copper were determined.
A copper concentrate containing arsenic-bearing copper minerals, or the so-called copper–arsenic concentrate, was supplied by Sumitomo Metal Mining Co., Ltd. (Tokyo, Japan). The copper–arsenic concentrate was a product of the rougher and cleaner flotation of Chilean copper ores. Upon receiving it, the copper–arsenic concentrate was immediately refrigerated in vacuum-sealed plastic bags to minimize oxidation.
Industrial-grade KAX (C6H11KS2O) from Quadra Chemicals Ltd. (Vaudreuil, Canada) was supplied by Sumitomo Metal Mining Co., Ltd. (Tokyo, Japan). Analytical-grade potassium hydroxide (KOH), hydrochloric acid (HCl), hydrogen peroxide (H2O2), and industrial-grade pine oil were purchased from Wako Chemical Industries (Tokyo, Japan). KAX, H2O2, and pine oil were used as the collector, oxidizing agent, and frother, respectively, whereas KOH and HCl were used as pH modifiers.
2.2 Characterization of the copper–arsenic concentrateThe size of the copper–arsenic concentrate was determined via wet sieving and categorized into six fractions: +104, −104 + 74, −74 + 53, −53 + 38, −38 + 22, and −22 µm. The size of each fraction was analyzed using X-ray fluorescence (XRF; ZSX Primus II, Rigaku, Japan). X-ray diffraction (XRD) measurements were performed using an Ultima4 X-ray diffractometer (Rigaku, Japan) with a Cu-Kα irradiation source to analyze the crystalline phase present in the copper–arsenic concentrate.
2.3 Flotation experimentsFigure 1 shows a schematic of the flotation experiments, which were carried out using a 670 mL Fahrenwald flotation cell. The copper–arsenic concentrate (220 g) was mixed with ultrapure water (370 mL) in the flotation cell and vigorously agitated for 60 min at 2200 rpm to disperse the aggregates. Similar agitation speeds were used for the oxidation treatment and flotation tests. The pulp density was reduced from 37% to 25% prior to the oxidation treatment. The oxidation treatment was carried out for 60 min using a H2O2 stock solution (30% w/w) with a final concentration of 0.1 M. The pH of the solution was immediately adjusted to the desired values (i.e., pH 9, 10, or 11) after the addition of H2O2. KAX (60 g/t) and pine oil (50 g/t) were added after the oxidation treatment. KAX and pine oil treatments were performed for 3 and 2 min, respectively. The desired pH value was maintained throughout the oxidation process and KAX and pine oil treatments.

Schematic of the flotation experiments.
Flotation tests were performed under a nitrogen environment (500 mL/min) to prevent oxidation during the tests. The froth layer was stabilized for 30 s before scraping for 3, 5, 7, and 15 min. Pine oil (50 g/t) was added to stabilize the froth layer after 15 min of flotation. The concentrates and tailings obtained were filtered, dried at 105°C for 12 h, and weighed. The recoveries of copper and arsenic were calculated using eq. (1), where xi,C and xi,T are the grades of metal i (i.e., copper or arsenic) in the concentrate and tailing, respectively, as analyzed by XRF. mC and mT are the masses of the concentrate and tailing, respectively. The Newton efficiency defined in eq. (2) was used to assess the separation selectivity between the non-arsenic copper and arsenic-bearing copper minerals. The recovery of non-arsenic copper was calculated by subtracting the recovery of copper associated with arsenic-bearing copper minerals from the recovery of total copper. The XRD pattern (Fig. 2) indicated that enargite was the main arsenic-associated mineral in the copper concentrate. Therefore, the arsenic recovery was assumed to be that of enargite to simplify the separation efficiency calculation.
| \begin{equation} \text{Recovery of metal i (%)} = \frac{\text{x}_{\text{i,C}}\text{m}_{\text{C}}}{\text{x}_{\text{i,C}}\text{m}_{\text{C}} + \text{x}_{\text{i,T}}\text{m}_{\text{T}}} \times 100\% \end{equation} | (1) |
| \begin{align} &\text{Newton efficiency}\text{ (%)} \\ &= \text{Recovery of arsenic-Recovery of non-arsenic copper} \end{align} | (2) |
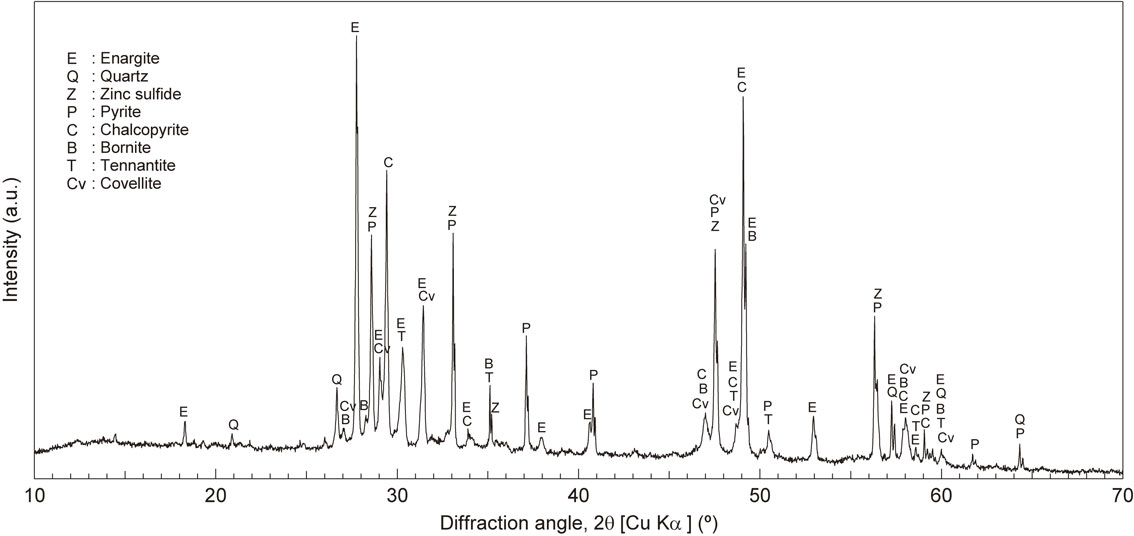
X-ray diffraction pattern of the copper–arsenic concentrate.
X-ray photoelectron spectroscopy (XPS) analysis was performed using an AXIS-ULTRA X-ray photoelectron spectrometer (Shimadzu-Kratos Co., Ltd., Manchester, UK) operated at 12 kV and 5 mA to investigate the effect of the oxidation treatment on the chemical species on the surface of the minerals. The copper–arsenic concentrate suspension was prepared by mixing 16.4 g of copper–arsenic concentrate with 50 mL of ultrapure water. The suspension obtained was agitated for 1 h. Subsequently, oxidation treatment was conducted for 1 h using 0.1 M H2O2 at pH 9. KAX treatment was then performed for 3 min by adding 60 g/t of KAX at pH 9. The treated copper–arsenic concentrate was filtered and freeze-dried for 3 h. High-resolution C 1s, Cu 2p, S 2p, and As 3d spectra were acquired and calibrated using the binding energy of C 1s at 284.6 eV.
2.5 Adsorption studyAn adsorption study was performed to investigate the effect of the oxidation treatment on the adsorption of KAX on the surface of copper sulfide and arsenic-bearing copper minerals. Pure enargite (Quiruvilca Mine, Santiago de Chuco, La Libertad, Peru) and chalcopyrite (Miyatamata Mine, Akita, Japan) were used for the adsorption study. These minerals were selected because they are the major minerals in the copper–arsenic concentrate. The pure minerals were ground using an agate mortar and pestle, dry-sieved, and passed through a 38 µm screener. A mineral suspension was prepared by mixing 0.3 g of mineral powder with 100 mL of ultrapure water. Oxidation treatment was then performed using 0.1 mM H2O2 at pH values of 9, 10, and 11 for 60 min. KAX treatment was conducted using 0.1 mM KAX for 3 min. Next, UV–vis absorbance was measured using a NanoPhotometerTM Pearl UV–vis spectrophotometer (Implen, Munich, Germany). The percentage of adsorbed KAX was calculated using eq. (3). C0 and Ct are the initial KAX absorbance and KAX absorbance at time t, respectively.
| \begin{equation} \text{KAX adsorption (%)} = \left(1 - \frac{\text{C}_{0}}{\text{C}_{\text{t}}} \right) \times 100\% \end{equation} | (3) |
Figure 2 shows the XRD pattern of the copper–arsenic concentrate. The XRD results indicated that the copper–arsenic concentrate mainly consisted of chalcopyrite (CuFeS2) as the main copper sulfide mineral, enargite (Cu3AsS4) as the main arsenic-bearing copper mineral, and pyrite (FeS2) and quartz (SiO2) as gangue minerals. In addition, bornite (Cu5FeS4), covellite (CuS), tennantite (Cu12As4S13), and zinc sulfide (ZnS) were identified by XRD. Table 1 shows that the copper–arsenic concentrate contains 31.0% copper and 3.9% arsenic. The size-by-size metal and mass distribution of copper–arsenic (Fig. 3) show that the copper–arsenic concentrate mostly consists of fine particles. Approximately 60% of the copper–arsenic concentrate was composed of fine particles with a size smaller than 38 µm. Approximately 63% arsenic and 64% copper were distributed in a size fraction of less than 38 µm.

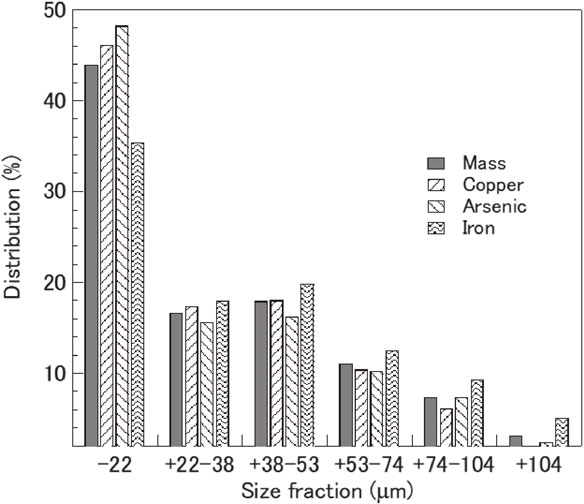
Size-by-size metal and mass distribution of the copper–arsenic concentrate (mass%).
Figures 4(A) and 4(B) show the recoveries of total copper and arsenic as a function of flotation time. Generally, these recoveries increased rapidly in the first 8 min of flotation and then reached a plateau profile after 15 min of flotation. For instance, the recoveries of total copper and arsenic were 16% and 20%, respectively, after 3 min of flotation at pH 9. The recoveries gradually increased to 29% for total copper and 34% for arsenic after 15 min of flotation at pH 9. An additional 15 min of flotation slightly improved the recoveries of total copper and arsenic to 38% and 42%, respectively. Therefore, 30 min of flotation was sufficient to collect all floatable minerals.

Recoveries of (A) total copper, (B) arsenic, and (C) non-arsenic copper. (D) Newton efficiency after treatment with 60 g/t of KAX, 0.1 M H2O2, and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9.
Figures 4(A) and 4(B) show that adding 60 g/t of KAX at pH 9 improved the final recoveries (after 30 min of flotation) from 38% to 55% for total copper and from 42% to 70% for arsenic, respectively. Moreover, Fig. 4(C) shows that the recovery of non-arsenic copper increased from 36% to 48% after the addition of 60 g/t of KAX. These results indicate that KAX improves the floatability of both copper sulfide and arsenic-bearing copper minerals. KAX is known to react with copper, forming cuprous amyl xanthate and dixanthogen, which improve the surface hydrophobicity of copper sulfide and arsenic-bearing copper minerals,18,19,24–26) thereby improving the floatability of these minerals.
The Newton efficiency presented in Fig. 4(D) indicates that the separation selectivity between copper and arsenic improved after KAX treatment at pH 9. This is due to the higher recovery of arsenic than that of non-arsenic copper (Figs. 4(B) and 4(C)). For instance, the Newton efficiency after KAX treatment reached a maximum value of 28% after 8 min flotation, showing a 21% improvement compared to that without KAX. The Newton efficiency then decreased with increasing flotation time.
The improvement in separation selectivity after KAX treatment may be attributed to the difference in the adsorption rate of KAX between copper sulfide and arsenic-bearing copper minerals. Therefore, KAX adsorption tests using enargite and chalcopyrite were performed under various pH conditions. As shown in Fig. 5, enargite adsorbed a higher amount of KAX than chalcopyrite after 3 min of adsorption at pH 9 without H2O2 treatment. The higher and faster adsorption of KAX on enargite likely caused the enargite to become more floatable than chalcopyrite. A previous study19) reported similar results, showing that arsenic-bearing copper minerals (enargite and tennantite) adsorbed KAX more rapidly than chalcopyrite at pH 9, making enargite and tennantite more floatable than chalcopyrite.

Effect of pH on the adsorption of KAX onto the surfaces of enargite and chalcopyrite with and without the H2O2 treatment. The initial concentration of KAX was 0.1 mM and the adsorption time was 3 min.
It was expected that the oxidation treatment using a 0.1 M H2O2 aqueous solution would reduce the floatability of copper and arsenic-bearing copper minerals. However, Fig. 4 shows that the recoveries of total copper and arsenic increased after oxidation treatment using a 0.1 M H2O2 aqueous solution. The recoveries of total copper and arsenic increased to 47% (Fig. 4(A)) and 68% (Fig. 4(B)), respectively, after 30 min of flotation. Remarkably, Fig. 4(C) shows that the oxidation treatment barely affected the recovery of non-arsenic copper. These results indicate that instead of reducing the recoveries of copper and arsenic, the oxidation treatment using a 0.1 M H2O2 aqueous solution improved the floatability of arsenic-bearing copper minerals. Furthermore, the optimum Newton efficiency was 38% after 8 min of flotation, which was improved by 30% compared to that without H2O2 treatment.
The improvement in separation selectivity was attributed to the presence of more floatable arsenic-bearing minerals. This is probably due to the formation of a metal-deficient surface of arsenic-associated minerals after the oxidation treatment. The S 2p spectra (Fig. 6) show that the H2O2 treatment caused the formation of polysulfide (Sn2−) and oxidized SO32− to SO42−. In addition, the H2O2 treatment produced Cu(OH)2, as indicated by the increasing intensity of the Cu(OH)2 species and decreasing intensity of the CuSO4 species in the Cu 2p spectra (Fig. 6).

High-resolution S 2p and Cu 2p spectra of the copper–arsenic concentrate after treatment with and without 0.1 M H2O2 and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9.
The presence of a metal-deficient surface, which is responsible for the higher floatability of the copper–arsenic concentrate (particularly arsenic-associated minerals), might be proven with the formation of polysulfide. The formation of a metal-deficient surface was reported9) as the main reason for floatable enargite during collectorless flotation under oxidizing conditions, with pulp potentials ranging from 91 to 541 mV versus Standard Hydrogen Electrode (SHE). In this study, the pulp potentials measured during the oxidation treatment were 300–330 mV versus SHE.
In addition to the metal-deficient surface, the As 3d spectra presented in Fig. 7 show that the As(V) peak disappeared and the As(III) peak appeared after the H2O2 treatment at pH 9. This phenomenon might be caused by As(V) dissolution under oxidizing conditions. The dissolution of As(V) and the formation of a cleaner surface might cause the arsenic-associated minerals to become less hydrophilic and, therefore, more floatable.

High-resolution As 3d spectra of the copper–arsenic concentrate after treatment with and without 0.1 M H2O2 and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9.
The addition of KAX (60 g/t) after oxidation treatment using a 0.1 M H2O2 aqueous solution significantly improved the recoveries of both copper and arsenic. The final arsenic recovery was 98%, whereas the final recoveries of non-arsenic copper and total copper were 64% and 76%, respectively. Figure 4(B) shows that the recovery of arsenic rapidly increased to 83% after 3 min of flotation. As shown in Fig. 4(C), the recovery of non-arsenic copper was 26%. The oxidation treatment likely enhanced the adsorption of KAX on arsenic-associated minerals, as shown in Fig. 5, thereby improving the floatability of these minerals. Enargite adsorbed 22% more KAX than chalcopyrite after oxidation treatment using 0.1 mM H2O2 at pH 9. These results correlate well with a previous study that showed that enargite and tennantite adsorbed KAX more rapidly than chalcopyrite after pre-oxidation using H2O2 aqueous solutions.19) Furthermore, the Cu(II) species formed after the oxidation treatment promoted the adsorption of KAX. Indeed, the Cu 2p spectra in Fig. 6 show that the intensity of Cu(OH)2 decreased slightly after H2O2 and KAX treatments compared to that after only H2O2 treatment. The lower intensity of the Cu(OH)2 species may be due to the reaction between Cu(OH)2 and xanthate, as shown in eqs. (4) and (5):
| \begin{equation} \text{Cu(OH)$_{2}$} + \text{2AX$^{-}$} \to \text{Cu(AX)$_{2}$} + \text{2OH$^{-}$} \end{equation} | (4) |
| \begin{equation} \text{Cu$^{2+}$} + \text{4AX$^{-}$} \to \text{Cu(AX)$_{2}$} + \text{(AX)$_{2}$} \end{equation} | (5) |
Figure 4(D) shows that the Newton efficiency was the highest (57%) after 3 min of flotation. The Newton efficiency then decreased as the recovery of non-arsenic copper gradually increased with increasing flotation time. The Newton efficiency was 34% after 30 min of flotation, which was slightly higher than that obtained after treatment with 60 g/t of KAX in the absence of H2O2 (33%). Despite similar Newton efficiencies, Fig. 8 clearly shows that adding 60 g/t of KAX after the oxidation treatment using 0.1 M H2O2 resulted in the lowest arsenic grade (0.16%) in the tailing, with a copper grade of 20.3% after 30 min of flotation. However, the arsenic level in the tailing was higher than 2% after treatment with 60 g/t of KAX or a 0.1 M H2O2 aqueous solution. These results indicate that oxidation treatment using H2O2 followed by the addition of KAX is considerably more effective in reducing the arsenic level in the tailing.

Grades of copper and arsenic in the tailing after treatment with 60 g/t of KAX, 0.1 M H2O2, and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9.
The Newton efficiency presented in Fig. 4(D) indicates that the separation selectivities of copper and arsenic-bearing copper minerals significantly improved after treatment with 60 g/t of KAX with and without the oxidation treatment using a 0.1 M H2O2 aqueous solution. Therefore, flotation experiments were performed under various pH conditions to evaluate the effect of pH on the flotation performance of copper concentrates containing arsenic-bearing copper minerals. Figure 9 shows the effect of pH on the recoveries of total copper, arsenic, and non-arsenic copper after treatment with 60 g/t of KAX with and without oxidation treatment using a 0.1 M H2O2 aqueous solution.

Effect of pH on the recoveries of total copper, arsenic, and non-arsenic copper and the Newton efficiency after 30 min of flotation. The copper concentrates containing arsenic-bearing copper minerals were treated with (A) 60 g/t of KAX and (B) a combination of a 0.1 M H2O2 aqueous solution and 60 g/t of KAX under various pH conditions.
Figure 9(A) shows that the recoveries of total copper, arsenic, and non-arsenic copper after treatment with 60 g/t of KAX increased with increasing pH. The recovery of arsenic significantly improved by 29% after increasing the pH value from 9 to 10. The recovery of arsenic was 99% after treatment with 60 g/t of KAX at pH 10. The recovery of non-arsenic copper after increasing the pH value from 9 to 10 increased by 17%, which is significantly lower than the improvement in arsenic recovery. This is likely due to the more rapid adsorption of KAX onto the surface of arsenic-bearing copper minerals compared to that onto the surface of copper sulfide minerals at a higher pH value (alkaline condition), as shown in Fig. 5. Although the KAX adsorption decreased with increasing pH, the amount of KAX adsorbed onto enargite remained higher than that onto chalcopyrite. The lower KAX adsorption with increasing pH (Fig. 5) is likely due to the lower stability of H2O2 at high pH values.27) The low stability of H2O2 reduced the amount of H2O2 available for oxidizing the surface at a high pH, resulting in a lower amount of oxidized copper available for the adsorption of KAX.
As shown in Fig. 9(A), the optimum conditions (33%) for separating copper sulfide and arsenic-bearing copper minerals using 60 g/t of KAX was at pH 10. The Newton efficiency decreased at pH 11. This is due to a higher recovery of non-arsenic copper and the maximum recovery of arsenic. Figure 10 shows that the arsenic level sharply decreased from 2.32% at pH 9 to 0.14% at pH 10 and 0.06% at pH 11 after treatment with 60 g/t of KAX and 30 min of flotation. These results indicate that producing a copper concentrate with a lower grade of arsenic, that is, lower than the penalty limit of 0.2%, is possible using the KAX treatment at the pH values of 10 and 11. These results demonstrate that KAX treatment under higher pH conditions can produce a “clean” copper concentrate with a lower arsenic grade. However, it should be noted that the grade of copper also decreased with increasing pH, that is, from 25% at pH 9 to 17% at pH 10 and 15% at pH 11.
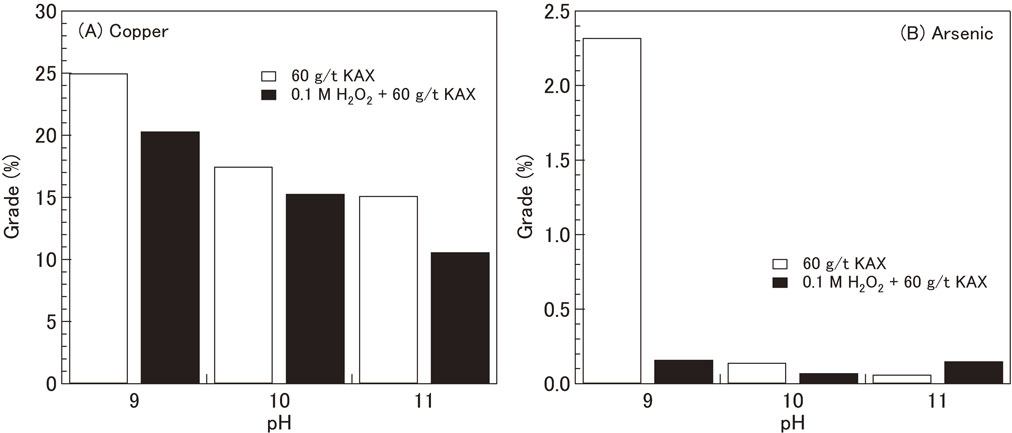
Effect of pH on the grades of (A) copper and (B) arsenic in the tailing after 30 min of flotation. The copper concentrates containing arsenic-bearing copper minerals were treated with 60 g/t of KAX and a combination of a 0.1 M H2O2 aqueous solution and 60 g/t of KAX under various pH conditions.
Figure 9(B) presents the effect of oxidation treatment using a 0.1 M H2O2 aqueous solution and adding 60 g/t of KAX under various pH conditions on the recoveries of total copper, arsenic, and non-arsenic copper and Newton efficiency. The recovery of arsenic reached its maximum after 30 min of flotation at pH 9. Therefore, increasing the pH had no significant effect on the recovery of arsenic. In contrast, the recovery of non-arsenic copper increased with increasing pH. Accordingly, the Newton efficiency decreased with increasing pH. For instance, the Newton efficiencies were 18% and 10% at pH values of 10 and 11, respectively. Furthermore, the grade of copper decreased from 20% at pH 9 to 15% at pH 10 and 10% at pH 11 (Fig. 10(A)). Meanwhile, the grade of arsenic decreased slightly from 0.16% at pH 9 to 0.07% and 0.15% at pH values of 10 and 11, respectively. These results indicate that oxidation treatment using H2O2, followed by the addition of KAX at a higher pH was less effective for separating copper and arsenic-bearing copper minerals.
3.4 Flotation kineticsThe flotation kinetics were evaluated using the classical first-order model shown in eq. (6), where R and Rm are the flotation recovery and maximum recovery for each metal after time t, respectively. k is the first-order kinetic rate constant,28–31) which can be used to evaluate the rate of a metal or mineral reaching the maximum recovery (Rm). Rm and k were derived by minimizing the sum of the squared errors between the experimental and calculated recoveries. Figure 11 shows the fitting results of eq. (6) and the experimental recovery data. All the results are in good agreement with the experimental data.
| \begin{equation} R = R_{m}(1 - e^{-kt}) \end{equation} | (6) |
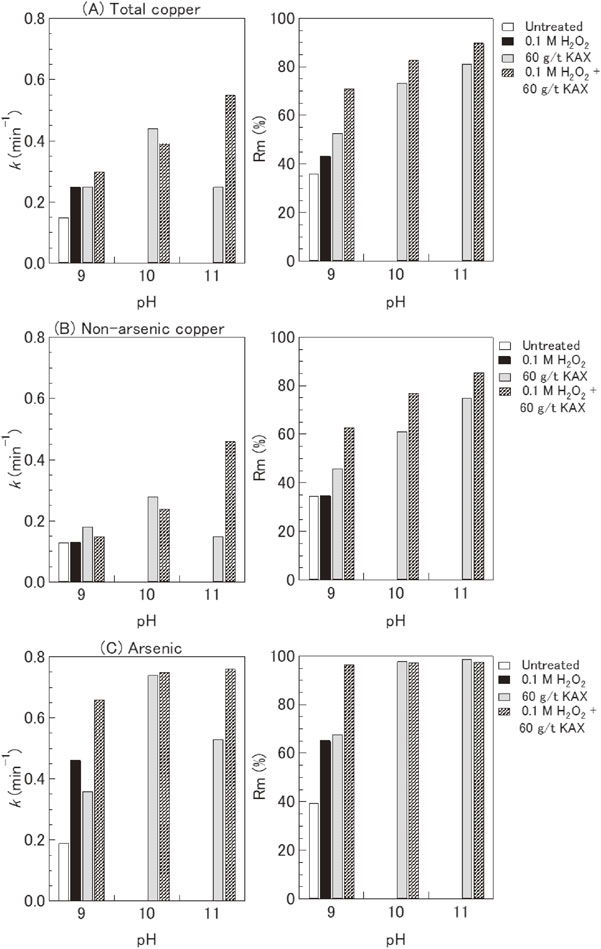
Flotation kinetics parameters (Rm (%) and k (min−1)) for total copper, non-arsenic copper, and arsenic under various treatment conditions.
Figure 11 shows that the k and Rm values for total copper, non-arsenic copper, and arsenic significantly increased after treatment with 60 g/t of KAX, 0.1 M H2O2, and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9. These results indicate that the minerals rapidly floated and reached maximum recovery after being subjected to these treatments. For instance, the constant k value for arsenic was 0.19 min−1 at pH 9, which significantly increased to 0.36, 0.46, and 0.66 min−1 after treatment with 60 g/t of KAX, 0.1 M H2O2, and a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9, respectively.
The Rm and k values of total copper, non-arsenic copper, and arsenic increased after raising the pH of a 60 g/t KAX aqueous solution from 9 to 10. Although the Rm value increased after treatment using 60 g/t of KAX at pH 11, the flotation rate decreased, as indicated by a lower k value. It is difficult to determine the exact cause of the lower k value at pH 11 because the rate constant is affected by many factors, such as mineral characteristics, reagents, and bubble particle interactions. Figure 11 shows that increasing the pH value in the treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX had a positive correlation with the flotation rate constant and the maximum recovery of the minerals. Both Rm and k values for all metal-associated minerals increased with increasing pH.
3.5 Proposed mechanism and processFigure 12 shows the correlation between the recoveries of arsenic and non-arsenic copper, which can be used to compare the effectiveness of each treatment condition for the separation of copper sulfide and arsenic-bearing copper minerals. Figure 12 shows that the treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX at pH values of 9, 10, and 11 selectively recovered arsenic-associated minerals with slight recovery of non-arsenic-associated minerals of copper in the beginning stage of flotation. However, the selectivity between arsenic and non-arsenic-associated minerals of copper decreased with increasing flotation time.

Flotation recoveries of arsenic and non-arsenic copper after treatments in KAX and H2O2 aqueous solutions under various pH conditions.
The mineralogical analysis of each flotation concentrate and tailing after the treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9 is presented in Fig. 13. The arsenic-bearing copper minerals (enargite and tennantite) were concentrated in the first 8 min (concentrates 1 and 2). In contrast, the copper sulfide minerals (chalcopyrite, bornite, and covellite) and pyrite were concentrated in the later stage (concentrates 3 and 4 and tailing).

XRD patterns of the flotation concentrates and tailing after treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9.
Based on the mineralogical analysis results, flotation kinetics, XPS analysis, and adsorption studies, we hypothesized that the main reason for the difference in flotation kinetics and selective flotation behavior is the difference in the surface abilities of copper sulfide minerals and arsenic-bearing copper minerals to adsorb KAX. The rapid adsorption of KAX on arsenic-bearing copper minerals causes these minerals to float faster than copper sulfide minerals, concentrating the arsenic-bearing minerals at the early stage of flotation. Oxidation treatment using H2O2 oxidizes the surface, enhancing the adsorption of KAX on the surface of arsenic-bearing minerals.
Notably, the treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9 exhibited a higher selectivity, that is, recovering a lower amount of non-arsenic copper, compared to the treatments using 60 g/t of KAX at pH values of 10 and 11. Indeed, the Newton efficiency (Fig. 9) and the final grades of copper and arsenic in the tailing (Fig. 10) indicate that the treatment using a combination of 0.1 M H2O2 and 60 g/t of KAX at pH 9 resulted in a better selectivity and cleaner copper concentrate (i.e., a lower arsenic content and higher copper grade) compared to other treatments.
Based on these results, we proposed a new process to separate copper and arsenic-bearing copper minerals via flotation, as shown in Fig. 14. Selective flotation produces a “dirty” copper concentrate, which contains copper with high-grade arsenic, and a “clean” copper concentrate, which contains copper with low-grade arsenic in the tailing. The “dirty” copper concentrate can be further processed to extract copper from arsenic-bearing copper minerals, whereas the “clean” copper concentrate can be processed in smelters.

Schematic of the proposed selective flotation process for the separation of arsenic-bearing copper minerals from copper concentrates using H2O2 oxidation treatment and KAX addition at pH 9.
In this study, the selective flotation of copper–arsenic concentrates using KAX and H2O2 oxidation treatment was investigated. The conclusions of this study are as follows:
This work was supported by Sumitomo Metal Mining Co., Ltd., Japan; Japan Oil, Gas, and Metals National Corporation (JOGMEC), Japan; and a Grant-in-Aid for Science Research (JSPS KAKENHI) from the Japan Society for the Promotion of Science (JSPS), Japan [Grant nos. JP23H03815, JP22K14636, JP22H00310, and JP19H02659]. This work was partly supported by the Advanced Research Infrastructure for Materials and Nanotechnology (No. JPMXP1222KU1020) sponsored by the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), Japan.