2021 Volume 62 Issue 5 Pages 341-344
2021 Volume 62 Issue 5 Pages 341-344
In order to elucidate the lifecycle of Coprinus comatus, we examined the number of nuclei in basidiospores, hyphal cells and oidia. Basidiospores isolated from the fruiting bodies of four Japanese strains were binucleate. In both primary and secondary mycelia, most of the cells were binucleate. In addition, oidia and oidiophores were observed for the first time in this mushroom and most of the oidia were binucleate. Based on these results, the lifecycle of C. comatus was inferred to be as follows. A homokaryotic binucleate basidiospore germinates and produces homokaryotic binucleate hyphae. After mating between compatible homokaryotic binucleate hyphae, a heterokaryotic binucleate secondary mycelium is produced. If environmental conditions are suitable for fruiting, homokaryotic binucleate basidiospores in the fruiting body are produced.
Coprinus comatus (O.F. Müll.) Pers. is a delicious and nutritious mushroom, and its extract have antioxidant properties (Badalyan, Gasparyan & Garibyan, 2003). Since few studies have examined aspects of the lifecycle of C. comatus, such as spore formation and karyological characterization of basidiosporogenesis (Chen, Shimomura, Yamaguchi, & Aimi, 2020c). In addition, young hyaline spores lack germ pores while mature black spores have germ pores. Using young spores and cow dung extract agar medium supplemented with 50 ppm (final concentration) of n-butyric acid, it is possible to isolate basidiospores of C. comatus (Chen et al., 2020a).
Regarding the mating type of this mushroom, some studies have reported that it is bipolar (Brunswick, 1924; Chen et al., 2020b), while others have reported that it is tetrapolar (Yu & Hui, 2008; Lin & Wu, 2012). However, in a previous study (Chen et al., 2020c), binucleate basidiospores in this mushroom were shown to be homokaryons. If we want to domesticate this mushroom, then we need to accurately clarify the complete lifecycle of this species. We therefore analyzed number of nuclei in basidiospores, mycelia and oidia in the Japanese strain of this mushroom. We also attempted to elucidate the complete lifecycle of C. comatus in order to develop and establish a breeding strategy for this mushroom.
Strains used in this study and culture conditionsIsolates (TUFC 30838 and TUFC 30228) were obtained from the Fungus/Mushroom Resource and Research Center, Faculty of Agriculture, Tottori University, Tottori, Japan. Isolates (NBRC 30325 and NBRC 30480) were obtained from the Biological Resource Center, National Institute of Technology and Evaluation, Tokyo, Japan.
The wild-type dikaryotic strain, TUFC 30838, was cultivated on a rice straw substrate using the method of Chen et al. (2020c). The fruiting body of C. comatus was harvested 8 d after the primordium had broken through the covered substrate and kept at room temperature. We followed the method of basidiospore isolation described by Chen et al. (2020a), and collected basidiospore isolates (2, 4, 7, 14, 15, 17, 25, 26, 31, 34, 35, 36, 41, 43, 44, 45, 46, 48, 49, 50) from fruiting bodies of TUFC 30838 for this study.
Microscopic observations of basidiospores and mycelia4’,6-diamidino-2-phenylindole (DAPI; Wako Pure Chemical Industries, Osaka, Japan) staining was used to observe basidiospores. Mature black basidiospores were collected from mature fruiting bodies and fixed in Carnoy’s solution (acetic acid:ethanol = 1:3, v/v). Samples were dehydrated under vacuum. After fixation, samples were centrifuged and Carnoy’s solution was removed. Then, 4 ppm of DAPI was added to the samples which were incubated at 4 °C for 2 d before nuclei were observed.
Slides were prepared to observe oidia and mycelia. Briefly, malt agar [MA; malt extract (20 g/L), agar (10 g/L), pH 8] was prepared and dropped it on a slide before it became solid. The agar on the slide was then inoculated with mycelia block to a slide. Slides were then incubated separately for 7 to 9 d at 25 °C in the dark. Slides were fixed in Carnoy’s solution and stained with 4 ppm DAPI, which stains nuclei, and 2 ppm Calcofluor White stain (Fluka, Buchs, Switzerland), which stains septa, overnight. Some preparations were then examined under a phase-contrast light microscope (Nikon Eclipse 50i; Nikon, Tokyo, Japan) and a light microscope (Nikon Eclipse 80i; Nikon) equipped with a digital camera (Nikon DS-L2; Nikon). Other preparations were examined under a fluorescence microscope (Nikon Eclipse 50i) fitted with a UV excitation apparatus (Nikon CSHG1, Nikon). The number of nuclei in 100 cells were then calculated for all samples.
To analyze the number of nuclei in individual cells in strain TUFC30838 and 20 of the basidiospore isolates obtained from fruiting bodies of strain TUFC30838, the mycelia were stained with both DAPI and Calcofluor White, and then observed under a fluorescent microscope. The results indicated that more than 60% of the cells in both tissue (Fig. 1A) and basidiospore (Fig. 1B) isolates were binucleate (Table 1). More than 70% of the oidia in TUFC 30838 and the basidiospore isolates 41 (A1 mating type) and 45 (A2 mating type) were uninucleate (Table 2). And this is the first report about production of oidiophores and oidia in C. comatus (Fig. 1C, D). One the other hand, we analyzed the number of nuclei in basidiospores in four tissue isolates TUFC 30838, TUFC 30228, NBRC 30325 and NBRC 30480. The results indicated that more than 70% of the basidiospores were binucleate (Fig. 1E; Table 2). Overall, the lifecycle of this mushroom can be described as follows. Basidia bear four basidiospores, each of which contains homokaryotic two nuclei (Chen et al., 2020c). Basidiospores germinate and develop into homokaryotic binucleate hyphae (primary hypha). When hyphal fusion between the hyphae of two primary mycelium carrying compatible mating genes occurs, the fused hyphae become heterokaryotic binucleate hyphae (secondary hyphae) (Chen et al., 2020b). This phenomenon is called mating, and after mating, secondary hyphae grow. Under suitable environmental conditions, the secondary hyphae form fruiting bodies which in turn produce basidia. After karyogamy and meiosis in each basidium, four homokaryotic binucleate basidiospores are produced (Fig. 2). In addition, the frequency of clamp formation at the septa that formed between the subterminal cell and the third cell of a mycelium was determined by the following formula: Clamp formation frequency (%) = (Number of septa with clamps/number of septa observed) × 100 that described by Chen et al. (2020b). The results showed heterokaryons and homokaryons can be distinguished by the frequency of clamp formation and couldn‘t find any pseudoclamp in neither heterokaryons nor homokaryons by microscopy. The frequency of clamp formation in heterokaryons was 30% or more, while that of homokaryons was 8% or less on initial pH 8 of malt agar (Chen et al., 2020b).

| Strain | Number of hyphal cellsa | ||||
| Polynucleate | Quadnucleate | Trinucleate | Binucleate | Uninuleate | |
| TUFC 30838 | 3 | 3 | 4 | 79 | 11 |
| 2 | 3 | 4 | 5 | 85 | 3 |
| 4 | 2 | 3 | 10 | 81 | 4 |
| 7 | 2 | 5 | 0 | 74 | 19 |
| 14 | 5 | 2 | 7 | 81 | 5 |
| 15 | 5 | 7 | 8 | 70 | 10 |
| 17 | 4 | 5 | 16 | 72 | 3 |
| 25 | 4 | 5 | 6 | 80 | 5 |
| 26 | 9 | 15 | 12 | 58 | 6 |
| 31 | 5 | 6 | 8 | 67 | 14 |
| 34 | 4 | 3 | 2 | 85 | 6 |
| 35 | 3 | 3 | 3 | 89 | 2 |
| 36 | 5 | 3 | 9 | 68 | 15 |
| 41 | 3 | 1 | 11 | 78 | 7 |
| 43 | 8 | 5 | 30 | 52 | 5 |
| 44 | 4 | 4 | 19 | 68 | 5 |
| 45 | 6 | 8 | 24 | 57 | 5 |
| 46 | 5 | 3 | 10 | 70 | 12 |
| 48 | 1 | 1 | 10 | 82 | 6 |
| 49 | 1 | 6 | 14 | 70 | 9 |
| 50 | 3 | 3 | 4 | 79 | 11 |
a Total observed cells in each sample were 100.
| Strain | Number of nuclei in oidia | ||||
| Total observed | Trinucleate | Binucleate | Uninuleate | DAPI-negative | |
| TUFC 30838 | 100 | 5 (5.0)a | 25 (25.0) | 70 (70.0) | 0 (0.0) |
| 41 | 100 | 4 (4.0) | 13 (13.0) | 83 (83.0) | 0 (0.0) |
| 45 | 100 | 7 (7.0) | 13 (13.0) | 80 (80.0) | 0 (0.0) |
| Strain | Number of nuclei in basidiospores | ||||
| Total observed | Trinucleate | Binucleate | Uninucleate | DAPI-negative | |
| TUFC 30838 | 1020 | 5 (0.5) | 852 (83.5) | 149 (14.6) | 14 (1.4) |
| TUFC 30228 | 469 | 0 (0.0) | 388 (82.7) | 42 (9.0) | 39 (8.3) |
| NBRC 30325 | 524 | 12 (2.3) | 392 (74.8) | 73 (13.9) | 47 (9.0) |
| NBRC 30480 | 865 | 9 (1.0) | 688 (79.5) | 51 (5.9) | 117 (13.5) |
a Numbers in parentheses indicate observation frequencies (%).
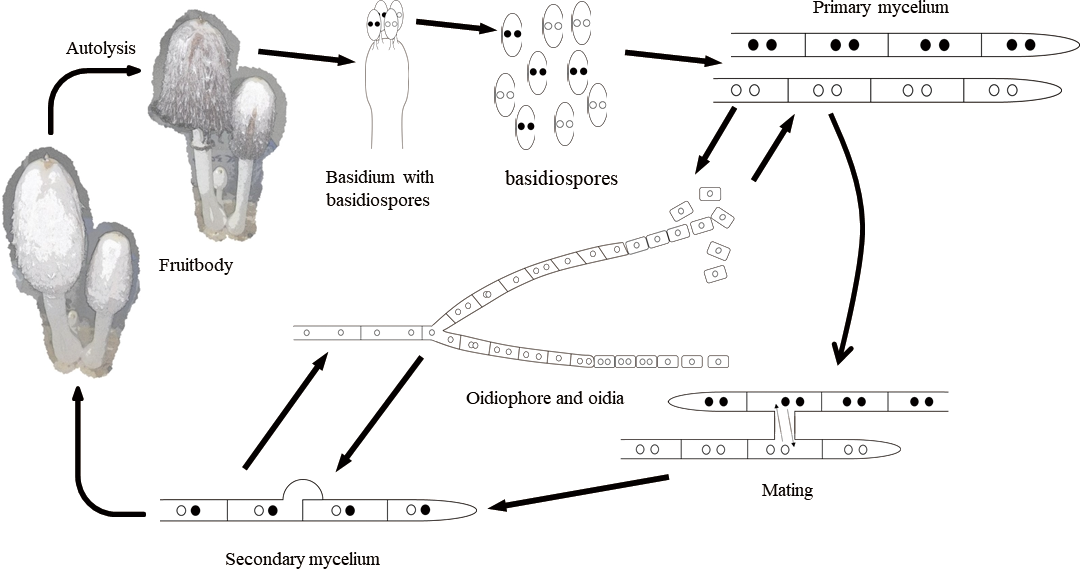
Basidia in C. comatus produced four basidiospores that described by Chen et al. (2020a). On the other hand, in a typical mushroom like Lentinula edodes (Berk.) Pegler, one basidium bears four uninucleate basidiospores (Shimomura, Kobayashi, Murakami, & Hasebe, 2011). However, binucleate and multinucleate basidiospores are also found in mushrooms; for example, binucleate basidiospores were produced in the basidia of Schizophyllum commune Fr. (Voelz & Niederpruem, 1964) and Coprinopsis cinerea (Schaeff.) Redhead, Vilgalys & Moncalvo (Kües, 2000). In different varieties of Agaricus bisporus (J.E. Lange) Imbach, tetranucleate or binucleate basidiospores have been observed in basidia (Kamzolkina et al., 2006). Therefore, we stained basidiospores from four strains of C. comatus from Japan, to investigate the existence of different mushroom varieties and lifecycle characteristics. Our results showed that binucleate basidiospores were produced in the basidia of all four Japanese strains of C. comatus. We therefore consider that these Japanese strains of C. comatus exhibit the same patterns of nuclear behavior during basidiosporogenesis. Homokaryotic binucleate spores were also observed in Helicobasidium mompa Nobuj. Tanaka (Aimi, Iwasaki, Kano, Yotsutani, & Morinaga, 2003) and Co. cinerea (Kües, 2000).
Based on our findings, we consider that homokaryotic mycelia only produce oidia with one kind of mating type, irrespective of whether the oidia are uninucleate or multinucleate. However, heterokaryotic mycelia can produce three types of oidia, i.e., homokaryotic A1 mating type oidia, and homokaryotic and heterokaryotic A2 mating type oidia. In secondary mycelia, as relatively few oidia were produced, more than 75% of the binucleate cells were observed, and abnormal dedikaryotizion was not observed. We therefore consider that, with respect to secondary mycelia, this mushroom is a stable binucleate heterokaryon.
Typically, homokaryotic and heterokaryotic mycelia are uninucleate and binucleate, respectively (Deacon, 2006). However, some exceptions have been reported in the literature. For example, in A. bisporus, the number of nuclei in mycelia ranges from three to five, depending on the variety (Kamzolkina et al., 2006). In addition, the primary mycelium of Kalaharituber pfeilii (Henn.) Trappe & Kagan-Zur and Morchella spp. is also multinucleate (Roth-Bejerano, Li, & Kagan-Zur, 2004; Volk & Leonard, 1990). In the C. comatus examined in the present study, both homokaryotic and heterokaryotic mycelia contained binucleate cells, which is similar to the case for Rhizopogon roseolus (Corda) Th. Fr. (Sawada et al., 2014) and Co. cinerea (Kües, 2000). Based on cytological studies of homokaryotic and heterokaryotic mycelia, the nuclear behavior and karyological development of all mushroom mycelia are not the same. For example, more than 30% of Agaricomycetous species have more than two nuclei in one heterokaryotic cell and clamp connections are irregular or absent (Boidin, 1971).
We attempted to elucidate the lifecycle of C. comatus from Japan based on the nuclear behaviors in the mycelium and basidium which are very important for breeding and transformation. We also found oidia, which will be very useful for isolating the homokaryon in further research. We hope that these findings may contribute to fundamental research on edible mushrooms. In future studies, we intend to investigate oidia and protoplast development in C. comatus for mutant selection.