2024 Volume 65 Issue 5 Pages 216-223
2024 Volume 65 Issue 5 Pages 216-223
Aspergillus species take up various metal ions from environment. The morphology of Aspergillus oryzae strains can vary under the influence of various metal ions. Here, the effects of Ti4+, V3+, Sr2+, Ba2+, Al3+, Fe2+, Zn2+, Mn2+, Ca2+, and Cu2+ on morphological parameters of A. oryzae strains RIB40 and RIB143 were estimated. Colony diameter, conidiation, vesicle head size, and stipe width in both strains varied with concentration. Ti4+, Sr2+, Ba2+, Al3+, Fe2+, and Ca2+ affected conidiation in similar tendency between two strains. The effects of Ti4+, V3+, Sr2+, and Ba2+ on the morphology of A. oryzae are reported here for the first time. Induction of growth of both strains by 0.0001% Ti4+ may help the fermentation industry. Induction of conidiation in RIB40 by 0.001% Cu2+ confirmed previous results that low concentrations of Cu2+ promote the growth of Aspergillus. The most novel finding is that 0.001% Zn2+ increased the vesicle head size in RIB40; possible reasons are discussed.
Minerals are released from rocks during soil formation via physical, chemical, and biological weathering processes (Hoffland et al., 2004; Kelly et al., 1998; Macheyeki et al., 2020). Physical weathering disintegrates the original primary minerals without changing their chemical composition, and chemical weathering converts them into secondary minerals (Macheyeki et al., 2020). Biological weathering dissolves minerals from rocks via acids secreted by organisms growing on the rocks (Hoffland et al., 2004; Kelly et al., 1998; Ribeiro et al., 2020). For instance, under favourable conditions, Aspergillus niger can secrete citric acid from its hyphal tips, to break down rock substrates (Hoffland et al., 2004), and thus constitutes an important component of the soil microbiota and ecosystems (Nayak et al., 2020).
Aspergillus species have high ability to take up various metal ions from the environment (Blatzer & Latgé, 2017; Ceci et al., 2012; Dusengemungu et al., 2020; Khani et al., 2012; Zhang et al., 2022). These metal ions play a significant role in the lives of filamentous fungi (Dusengemungu et al., 2020). For instance, Fe2+ takes part in vital biological mechanisms as a component of the electron transport chain, amino acid metabolism, and biosynthesis of DNA; Cu2+ and Zn2+ are incorporated into superoxide dismutase to protect against reactive oxygen species; Ca2+ belongs to cell wall functional groups; Mn2+ is essential for the growth of microorganisms (Bakti et al., 2018; Fejes et al., 2020; Haas, 2012; Latha et al., 2012). However, excessive Fe2+, Cu2+, Zn2+, Ca2+, or Mn2+ promote the formation of reactive oxygen species and damage cells (Haas, 2012; Krumova et al., 2016). Metal homeostasis in cells of Aspergillus is critical for its growth. The various environmental factors, including metal ions, may influence morphological forms in Aspergillus species.
Aspergillus oryzae can be isolated from plants, foods, and soils (El-Korany et al., 2020; Machida et al., 2008; Sahnoun et al., 2012). Various strains are widely used for production of fermented foods, enzymes, and secondary metabolites (Chang et al., 2014; Gomi, 2014; Nigam & Singh, 1999). A. oryzae can be classified into three groups (1-3) depending on the degree of deletion of aflatoxin biosynthesis genes (Kusumoto et al., 2000). Group 1 strains have few deletions while group 3 strains have deletion of the most of aflatoxin biosynthesis homologs (Kusumoto et al., 2000). It was supposed that group 1 strains are less domesticated than group 3 strains due to the less deletion degree of aflatoxin biosynthesis homologs (Kusumoto et al., 2000; Liu et al., 2023; Watarai et al., 2009). This different tendency of gene deletion between group 1 and group 3 strains may give different responses of two group strains to soil components (Liu et al., 2023).
We have found that one of soil components, humic acid stimulates or inhibits the growth of A. oryzae strains (Liu et al., 2023). Metal ions chelated by humic acid differentially affected giant colony diameter, stimulating it in strain RIB40 (Group 1) but not in strain RIB143 (Group 3), alone or mixed together (Liu et al., 2023). It was speculated that the chelated metal ions partly cause the differences in morphological response to humic acid in the strains. Al3+, Ca2+, Ti4+, V3+, Mn2+, Fe2+, Sr2+, and Ba2+ are detected in commercial humic acid as chelated metal ions (Liu et al., 2023). Furthermore, it was accidently found that a low concentration of Cu2+ increased conidiation in RIB40 and deepened the conidial color in RIB143, and that Zn2+ extended the aerial hyphae in RIB40 (data not shown). These ten types of metal ions may affect morphological characters in A. oryzae. To evaluate the growth and morphological changes of A. oryzae in the presence of various metal ions, giant colony diameter, conidial number, vesical head size, and stipe width of RIB40 and RIB143 under different concentrations of Ti4+, V3+, Sr2+, Ba2+, Al3+, Fe2+, Zn2+, Mn2+, Ca2+, and Cu2+ were determined.
Strains RIB40 and RIB143 were obtained from the National Research Institute of Brewing, Higashi-Hiroshima, Japan. Both were grown in Czapek-Dox Agar (CDA) medium (Difco Laboratories Inc., Franklin Lakes, NJ, USA; 30 g/L sucrose, 3 g/L NaNO3, 1 g/L K2HPO4, 0.5 g/L MgSO4, 0.5 g/L KCl, 0.01 g/L FeSO4) in 2% (w/v) agar. Water used for the media was pre-treated by reverse osmosis. After 13 d growth at 25 °C, conidia were collected by gently scraping the plate surface with a sterilized cell spreader in 0.8% (w/v) NaCl plus 0.1% (w/v) Tween 80. Following filtration at sterilized condition, the conidial suspension was washed with 0.1% (w/v) Tween 80 and stored in 25% (w/v) glycerol at −80 °C for later inoculation.
2.2. Addition of metal ions to growth mediumTi4+, V3+, Sr2+, Ba2+, Al3+, Fe2+, Zn2+, Mn2+, Ca2+, and Cu2+ were added individually to CDA medium at different concentrations (Table 1) before autoclaving. In the case of Fe2+, the additional concentrations were separately indicated from original Fe2+ in CDA medium. The pH of the medium was adjusted to that of the control (CDA medium without the addition of metal ions) with 1 M KOH (Nacalai Tesque, Inc., Kyoto, Japan).
| Metal ion name | Symbol | Added chemical | Company information of chemical | Concentration (w/v) | ||||
| Titanium | Ti4+ | Ti(SO4)2 | Kanto Chemical Co., Inc., Tokyo, Japan | 0%, | 0.0001%, | 0.001% | ||
| Vanadium | V3+ | V(NO3)3 | Kanto Chemical Co., Inc., Tokyo, Japan | 0%, | 0.001%, | 0.01%, | ||
| Strontium | Sr2+ | Sr(NO3)2 | Nacalai Tesque, Inc., Kyoto, Japan | 0%, | 0.001%, | 0.01%, | 0.1% | |
| Barium | Ba2+ | Ba(NO3)2 | Nacalai Tesque, Inc., Kyoto, Japan | 0%, | 0.0001%, | 0.001%, | 0.01% | |
| Aluminum | Al3+ | Al(NO3)3 | Kanto Chemical Co., Inc., Tokyo, Japan | 0%, | 0.001%, | 0.01%, | ||
| Iron | Fe2+ | FeSO4 | Nacalai Tesque, Inc., Kyoto, Japan | 0%, | 0.001%, | 0.01%, | 0.1% | |
| Zinc | Zn2+ | ZnSO4 | Nacalai Tesque, Inc., Kyoto, Japan | 0%, | 0.00001%, | 0.0001%, | 0.001%, | 0.01% |
| Manganese | Mn2+ | Mn(NO3)2 | Wako Pure Chemical Industries, Ltd., Osaka, Japan | 0%, | 0.001%, | 0.01%, | 0.1% | |
| Calcium | Ca2+ | CaCl2 | Wako Pure Chemical Industries, Ltd., Osaka, Japan | 0%, | 0.001%, | 0.01%, | 0.1% | |
| Copper | Cu2+ | CuSO4 | Wako Pure Chemical Industries, Ltd., Osaka, Japan | 0%, | 0.001%, | 0.01%, | 0.1% | |
Conidial stock solutions of RIB40 and RIB143 in glycerol were spread with a sterilized cotton swab on CDA medium and incubated at 30 °C for 14 d in the dark. Conidial suspensions were collected as in section 2.1., counted with a hemocytometer under a microscope (Optiphot-2, Nikon, Tokyo, Japan), and then diluted in sterilized Milli-Q water to 103 conidia/µL. 5 µL of suspension (containing 5 × 103 conidia) was spotted on the center of the plate and held the plate for 7 d at 30 °C. After incubation, the diameter of each giant colony was determined in four directions on day 7 and averaged. Conidia were collected as in section 2.1. and directly counted under a microscope (Optiphot-2) without filtration. Conidial density was calculated as conidial number per colony area = conidial number / π (average colony diameter / 2)2 and presented as conidial number per mm2; conidial number was presented as conidial number per plate.
2.4. Determination of vesical head size and stipe widthVesicle head size and stipe width of aerial hyphae in slide cultures were determined. In brief, RIB40 and RIB143 were inoculated on the side of a square of CDA agar with or without metal ion supplementation (3 mm × 3 mm × 2 mm high) by a sterilized toothpick. The agar was set on a micro-slide glass (Matsunami Glass Ind., Ltd., Osaka, Japan) and covered with a micro-cover glass (Matsunami Glass) and held in a sterilized plate at 30 °C under 96%-99% relative humidity. The humidity was maintained by putting containers of water in the incubator, and measured by digital thermo-hygrometer (Waves Inc., Tokyo, Japan). After 3 d, a drop of lactophenol cotton blue stain (Muto Pure Chemical Co., Ltd., Tokyo, Japan) was placed on a clean micro-slide glass and carefully covered with the micro-cover glass removed from the agar. Slides were examined under a light microscope (Optiphot-2), and the vesicle head size and stipe width of the aerial hyphae (Supplemental Fig. S1) were determined with ToupView software (Hangzhou ToupTek Photonics, Zhejiang, China).
2.5. Statistical analysisThe results of 3 independent replicates for giant colony diameter, conidial density, and conidial number were analysed by two-tailed Student's t-test, and the relative values of three parameters were calculated as the ratio of treatment to control. The results of 10 records per slide for vesicle head size and stipe width were analysed by Tukey's test in an online box plot tool (BoxPlotR: http://shiny.chemgrid.org/boxplotr/). Significant differences were indicated as *P < 0.05, **P < 0.01, and ***P < 0.001. In bar graphs, values are presented as mean ± standard error (SE) of 3 replicates. In boxplot graphs, whiskers depict maximum and minimum without outliers, and the box depicts median, first, and third quartiles in 10 records of each slide. Dots are outliers.
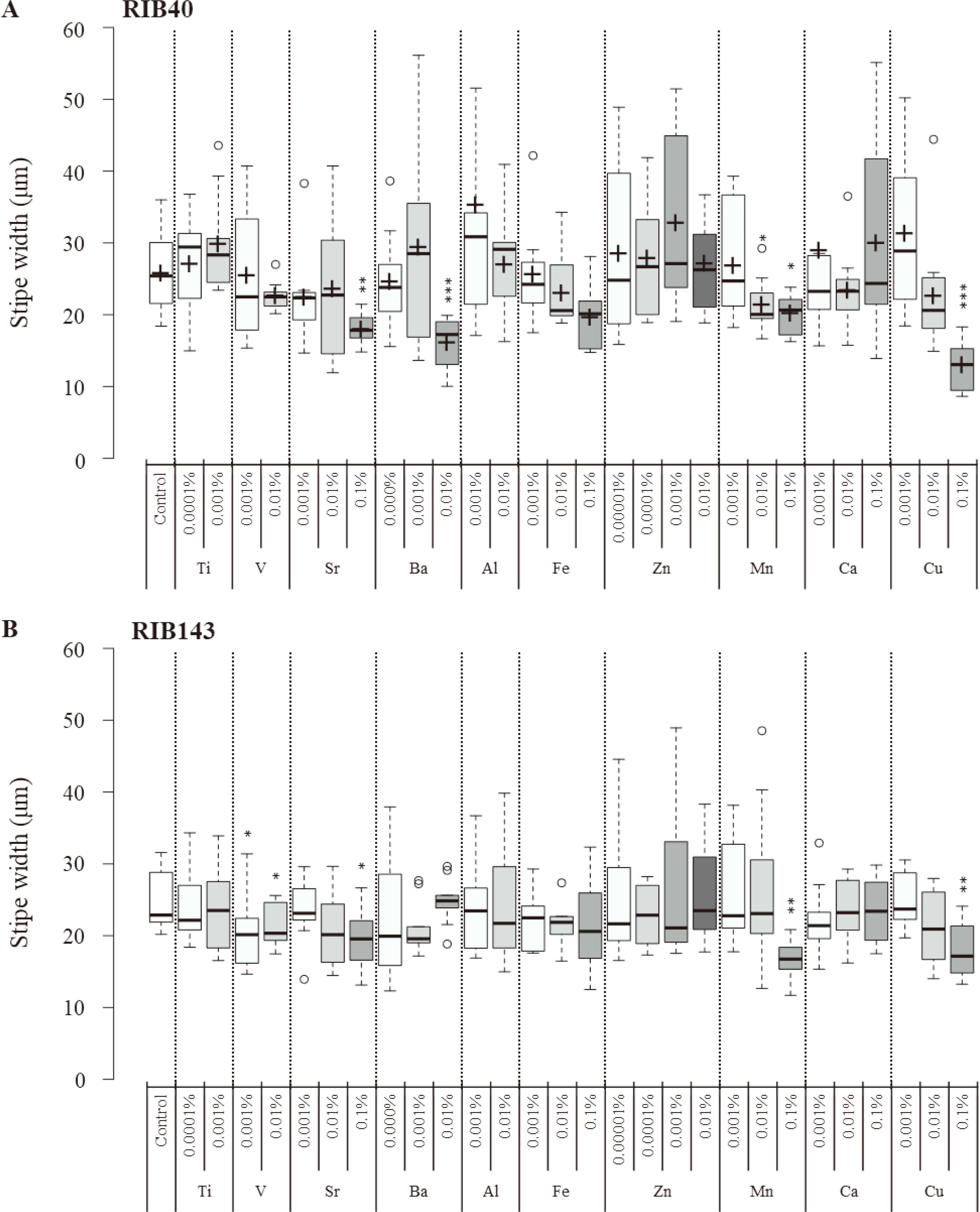
The effects of 10 metal ions at two to four concentrations each on the vegetative growth, reproductive capacity, and morphological changes of A. oryzae strains RIB40 and RIB143 were tested. The vegetative growth capacity was assessed by giant colony diameter, reproductive capacity was assessed by conidiation (conidial number), and morphological change was assessed by vesicle head size and stipe width of conidiophores responding to metal ions. Low to moderate concentrations of individual metal ions brought different effects on giant colony diameter (Fig. 1; Supplemental Figs. S2, S3), conidiation (as conidial number; Fig. 2), vesicle head size (Fig. 3), and stipe width of aerial hyphae (Fig. 4).



Studies involved in Ti4+ and Aspergillus species mostly focused on the photocatalytic activity of TiO2 (nanoparticle) (Babaei et al., 2016; Yang et al., 2023), and TiO2 inhibited the growth of Aspergillus flavus (closely related species of A. oryzae) at 0.1% with UV irradiation (Babaei et al., 2016). However, direct effect of Ti4+ on the growth of A. oryzae is not known. The stimulative effect of 0.0001% Ti4+ on the vegetative growth and conidial number (Figs. 1, 2) of A. oryzae (RIB40 and RIB143) here is the first finding.
The removal of V3+ and Sr2+ from the environment by Aspergillus species is a common focus in studies (Pan et al., 2009; Mirazimi et al., 2015; Rasoulnia & Mousavi, 2016), and no information about related studies between Aspergillus species and Ba2+, even this metal is the 14th most abundant element in Earth's crust (Cappuyns, 2018). The effect of V3+, Sr2+ and Ba2+ on the morphological characteristics of A. oryzae is not known. The stimulative effects of 0.01% V3+ and 0.01% Sr2+ on the vegetative growth capacity of RIB40 (Fig. 1A), 0.1% Sr2+ on the vegetative growth capacity of RIB143, and stimulative effects of 0.001% V3+ on the reproductive capacity in RIB40 (Fig. 2B) were the first findings. Other concentrations of V3+ and Sr2+ inhibited or unaltered on the vegetative and reproductive capacities in RIB40 or RIB143 (Figs. 1, 2). All tested concentrations of Ba2+ stimulated the vegetative growth capacity in RIB40, whereas 0.01% Ba2+ inhibited it in RIB143 (Fig. 1). Additionally, 0.001 % and 0.01 % Ba2+ inhibited the reproductive capacity in both strains (Fig. 2).
Al3+ occurs commonly in acidic soils (He et al., 2019). It was reported that A. oryzae (isolated from the River Nile, Luxor, Egypt) showed tolerance against 0.0001% to 0.1% of Al3+ (Mahmoud et al., 2017). In this study, 0.001% Al3+ stimulated the vegetative growth of RIB40 (Fig. 1A), and instead, 0.01% Al3+ decreased the vegetative growth capacity and reproductive capacity in RIB40 and RIB143 (Figs. 1, 2). Here is the first to report about reproductive capacities of Aspergillus species at different concentrations of Al3+. Our results of the vegetative growth and conidiation capacities of both strains under different concentrations of Ti4+, V3+, Sr2+, Ba2+, and Al3+ may provide useful information for further study of the effects of these metal ions in Aspergillus species.
Fe2+ is a critical micronutrient for the growth and survival of most microorganism (Frawley & Fang, 2014; Haas, 2012). The tolerance of the A. oryzae strain to the concentrations of Fe2+ from 0.0001% to 0.01% was reported (Mahmoud et al., 2017). In the present result, the additional 0.001% Fe2+ stimulated the vegetative growth capacity of RIB40 and RIB143, but the additional 0.1% reduced it (Fig. 1). The reason of the stimulative effect of 0.001% Fe2+ on the growth of RIB40 and RIB143 should be further analysed. Excessive Fe2+ has potential to promote the formation of reactive oxygen species and damage cells (Haas, 2012). The reason of the inhibitive effect of 0.1% Fe2+ on the growth of two strains may be the over-production of relative oxygen species in the cells. The yellow color of CDA medium containing 0.1% Fe2+ is the original color of the medium before inoculating the strains and may be the one of ferric oxide.
Zn2+ is the second most abundant transition micronutrient required by microorganisms and is essential for biochemical processes, cellular growth, and development (Amich & Calera, 2014). Hartikainen et al. (2012) reported that Zn2+ at 100, 200 and 400 mg/kg (0.01 - 0.04%) (contained on malt extract agar plate) decreased the most growth of some saprotrophic fungi (Basidiomycete, Ascomycete and Zygomycete) (Hartikainen et al. 2012). The only exception within ascomycete and zygomycete was the increased growth of Coniothyrium sp. with the same concentration of Zn2+ (Hartikainen et al. 2012). They also described that several Basidiomycete fungi increased their growth with the same concentration of Zn2+. In this study, most concentrations of Zn2+ inhibited the vegetative capacity in RIB40 and RIB143, and it showed a concentration-independent tendency (Fig. 1). In addition, Lanfranco et al. (2002) reported that Zn2+ affected the growth and morphology of the ericoid symbiotic fungus, led to apical swellings, and increased branching in the sub-apical parts. The length of aerial hyphae of RIB40 increased by Zn2+, and the colony of RIB40 was dense and fluffy along with increasing Zn2+ concentration (Supplemental Fig. S2). The mycelial dense within the colony of RIB143 was observed as well, but fluffy was not observed in RIB143 responding to Zn2+. RIB40 may change its hyphal fluffiness responding to Zn2+, besides RIB143 may not, probably due to the difference of the gene set.
Mn2+ are essential for the growth of microorganisms (Fejes et al., 2020). Mn2+ from 0.0001% to 0.1% unaltered the growth of A. oryzae strain (Mahmoud et al., 2017). However, in this study, Mn2+ gradually stimulated the vegetative growth capacity from 0.001% to 0.01%, and sharply inhibited it at 0.1% in two strains (Fig. 1). In addition, Mn2+ (0.001%, 0.01%, and 0.1%) decreased conidial number, however, 0.1% Mn2+ recovered conidial density because of the serious reduction of giant colony diameter in RIB40 (Figs. 1A, 2A; Supplementary Fig. S4).
Binding of Ca2+ to cell wall functional groups as a macronutrient supports the growth of microorganisms (Latha et al., 2012). For instance, Ca2+ affects growth, hyphal morphology, and citric acid production in A. niger (Pera & Callieri, 1997). Here, Ca2+ stimulated colony growth at 0.001% and significantly at 0.01% in RIB40, and at 0.01% and 0.1% in RIB143 (Fig. 1; Supplemental Figs. S2, S3). However, significantly inhibited reproductive capacity (Fig. 2; Supplemental Fig. S4) at 0.01% and 0.1%. Therefore, Ca2+ stimulated hyphal growth but inhibited reproductive capacity. The reason of stimulative effects of Ca2+ on hyphal growth was not clear. The inhibitive effects of Ca2+ on reproductive capacity might be due to reduced expression of some Ca2+ transporters which involved in asexual conidiation (Wang et al., 2021).
Cu2+ is a cofactor of antioxidant enzymes such as laccase and superoxide dismutase (Raffa et al., 2019). Hartikainen et al. (2012) reported that most of the 18 strains of fungi decreased their growth with 100-400 mg/kg (0.01-0.04%) of Cu2+, with the exception of one Basidiomycete. In A. oryzae, Cu2+ regulates conidiation via Cu2+-dependent superoxide dismutase and induces conidiation (Katayama & Maruyama, 2023). Therefore, incorporation of Cu2+ into superoxide dismutase to act against reactive oxygen species may explain the induction of conidiation in RIB40 by 0.001% Cu2+. Excessive Cu2+ (0.1%) may have caused intercellular toxicity and the inhibition of growth and conidiation in RIB40 and RIB143 (Figs. 1, 2). This toxic effect of 0.1% Cu2+ on growth of filamentous fungi is also observed in A. fumigatus (Krumova et al., 2016).
3.2. Effects of metal ions on vesical head size and stipe widthHigh concentrations of V3+ (0.01%), Mn2+ (0.1%), and Cu2+ (0.1%) decreased vesical head size in RIB40 and RIB143 (Fig. 3). High concentration of Ba2+ (0.01%), Fe2+ (0.1%), and moderate concentration of Mn2+ (0.01%) only decreased vesical head size in RIB40 (Fig. 3A). High concentrations of Sr2+ (0.1%), Mn2+ (0.1%), and Cu2+ (0.1%) decreased stipe width in RIB40 and RIB143 (Fig. 4). High concentration of Ba2+ (0.01%) and moderate concentration of Mn2+ (0.01%) only decreased stipe width in RIB40 (Fig. 4A). High concentrations of V3+ (0.001%, 0.01%) only decreased stipe width in RIB143 (Fig. 4B). Other metal ions did not affect vesical head size and stipe width in RIB40 or RIB143 at any concentrations (Figs. 3, 4).
Specially, 0.001% Zn2+ stimulated vesicle head size in RIB40 (Fig. 3A; Supplemental Fig. S5). The BrlA transcription factor is known to control the initiation of aerial hyphal development, including development of vesicles, metuale, phialides, and conidia (Yu, 2010). Zn2+ is important for forming a C2H2 zinc finger DNA-binding domain in BrlA (Wu et al., 2018). It is bold to suppose that the addition of 0.001% Zn2+ to growth medium disturbed the regulation network of brlA and further affected vesicle head size in RIB40. One explanation for the stimulation of vesicle head size by 0.001% Zn2+ is that some enzymes or signalling proteins involved in the development of the vesicle head may interact with zinc, i.e., having a zinc binding protein in the case of RIB40 but not in RIB143. Clarifying the genetic differences between RIB40 and RIB143 may identify genes related to vesicle head development in A. oryzae. Our findings may contribute new tools with which to clarify the mechanism of conidial head development in fungi.
Overall, the addition of metal ions to CDA medium was separated to six sets (Ti4+ and Ba2+, V3+ and Sr2+, Al3+ and Fe2+, Ca2+ and Cu2+, Zn2+, Mn2+), due to the limitation of the incubation space. The relative value of each parameter in Fig 1 and Fig. 2 was calculated as the ratio of treatment to control. Therefore, the results of relative value were not affected by the true values of each parameter in Fig. 1 and Fig. 2. Ti4+, Fe2+, Mn2+, and Cu2+ affected the relative value of colony diameter (Fig. 1); Ti4+, Sr2+, Ba2+, Al3+, Fe2+, and Ca2+ affected the relative value of conidial number (Fig. 2); V3+ and Cu2+ affected vesicle head size (Fig. 3); and Sr2+ and Cu2+ affected stipe width (Fig. 4) in similar tendency between two strains. However, V3+, Sr2+, Ba2+, Al3+, Mn2+, Ca2+, and Cu2+ affected morphology more in RIB40 than in RIB143. Domestication modifies morphological, physiological, and genetic characteristics (Steensels et al., 2019). According to the degree of aflatoxin biosynthesis homologs gene deletion, RIB143 is thought to be more domesticated strain than RIB40 (Kusumoto et al., 2000), and thus the mutation of genes in RIB143 during its domestication may explain the difference in morphology between RIB143 and RIB40 in response to these metal ions. It was supposed that the gene functions sensing or responding to metal ions in the environment may have more disappeared or weakened in RIB143 during the domestication from environmental strain. It should be interesting to identify and investigate some genes or signal pathways causing the different response to A. oryzae strains to these metal ions.
This study was conducted to estimate the influence of varied metal ions on the morphology of two A. oryzae strains, RIB40 and RIB143. This study firstly reported the morphology of RIB40 and RIB143 responding to Ti4+, V3+, Sr2+, Ba2+, and Zn2+. Increased growth of two strains by 0.0001% Ti4+ is a novel point, while increased vesicle head size in RIB40 by 001% Zn2+ provided the most novel finding in this study.
All authors declare no conflicts of interest.
This study was supported by the Institute for Fermentation, Osaka, Japan, under grant number K-2021-008.