2023 Volume 10 Pages 163-168
2023 Volume 10 Pages 163-168
A 61-year-old man presented with massive epistaxis, amaurosis, nausea, and severe headache. A detailed examination revealed a subarachnoid hemorrhage and prolactinoma. Angiography showed a small internal carotid artery pseudoaneurysm and inadequate collateral circulation; thus, uncomplicated coil embolization was performed. Considering the side effects of medication, such as cerebrospinal fluid rhinorrhea, the patient was followed up for asymptomatic prolactinoma without medication after discharge. At 40 months later, aneurysm recurrence was confirmed. Flow diverter device placement was performed, and the outcomes were excellent. In the present report, we described a rare case of a ruptured internal carotid artery aneurysm in an untreated prolactinoma and discussed the literature.
Internal carotid artery (ICA) pseudoaneurysm (ICAPA) is one of the rarest but well-recognized complications of transsphenoidal surgery. This complication is reported in 0.2%-1.4% of the patients undergoing transsphenoidal surgery.1-4) ICAPAs can subsequently result in other complications, such as subarachnoid hemorrhage (SAH), epistaxis, and carotid-cavernous fistula, leading to consequences, such as permanent neurological deficits or death. Prolactinoma is the most common subtype of functioning pituitary adenomas (PAs); it represents an important cause of hypogonadism and infertility.5) Because of their relatively higher invasive characteristics, sellar lesions should be completely eliminated. Several cases of cerebrospinal fluid (CSF) rhinorrhea or aneurysm rupture caused by the rapid shrinkage of adenoma following medication administration has been reported.6-9) However, no cases of SAH and epistaxis in completely untreated prolactinomas have been reported. In this report, we present a rare case of a ruptured ICAPA with simultaneous occurrence of SAH and massive epistaxis in an untreated prolactinoma. Furthermore, we conducted a literature review.
A 61-year-old man was immediately admitted with complaints of massive epistaxis, amaurosis fugax, nausea, and severe headache. Computed tomography (CT) revealed SAH and a tumor in the sphenoid sinus (Fig. 1A). CT angiography (CTA) showed the presence of a small right ICA paraclinoid aneurysm and partial destruction of the bony structures of the skull base (Fig. 1B and C). Coronal T2-weighted magnetic resonance imaging (MRI) and coronal CTA revealed that the large pituitary tumor and aneurysm were adjacent to each other (Fig. 1B and D); the aneurysm was located at the boundary between the cisternal space and sphenoid sinus. Cerebral angiography, including balloon test occlusion (BTO), was performed under general anesthesia. These examinations demonstrated an aneurysm with an irregular shape and a diameter of 2.9 mm (Fig. 1E and F) and the presence of a collateral flow via the anterior communicating artery. Moreover, BTO showed nonsynchronous venous filling on the occluded side. Coil embolization was performed after obtaining sufficient written consent. Considering the acute ruptured status and the possibility of recurrence, coils were simply inserted without stents. Digital subtraction angiography (DSA) revealed a neck remnant (Fig. 2A).

Clinical images after the onset of subarachnoid hemorrhage and epistaxis.
(A) Coronal image of plain head computed tomography (CT). The hematoma was distributed around the pituitary tumor periphery and right Sylvian fissure. (B) Coronal image of contrast-enhanced CT. The white arrow indicates a small ruptured aneurysm embedded in the tumor. (C) CT bone image showed destruction of the surrounding skull base, including the sella base. (D) Coronal image of Gd-contrast T1-weighted image. A large pituitary tumor was enhanced. (E and F) Right internal carotid angiography and three-dimensional volume rendering image show a small irregularly shaped aneurysm of approximately 2 mm with a bleb at the same site as in Fig. 1B.
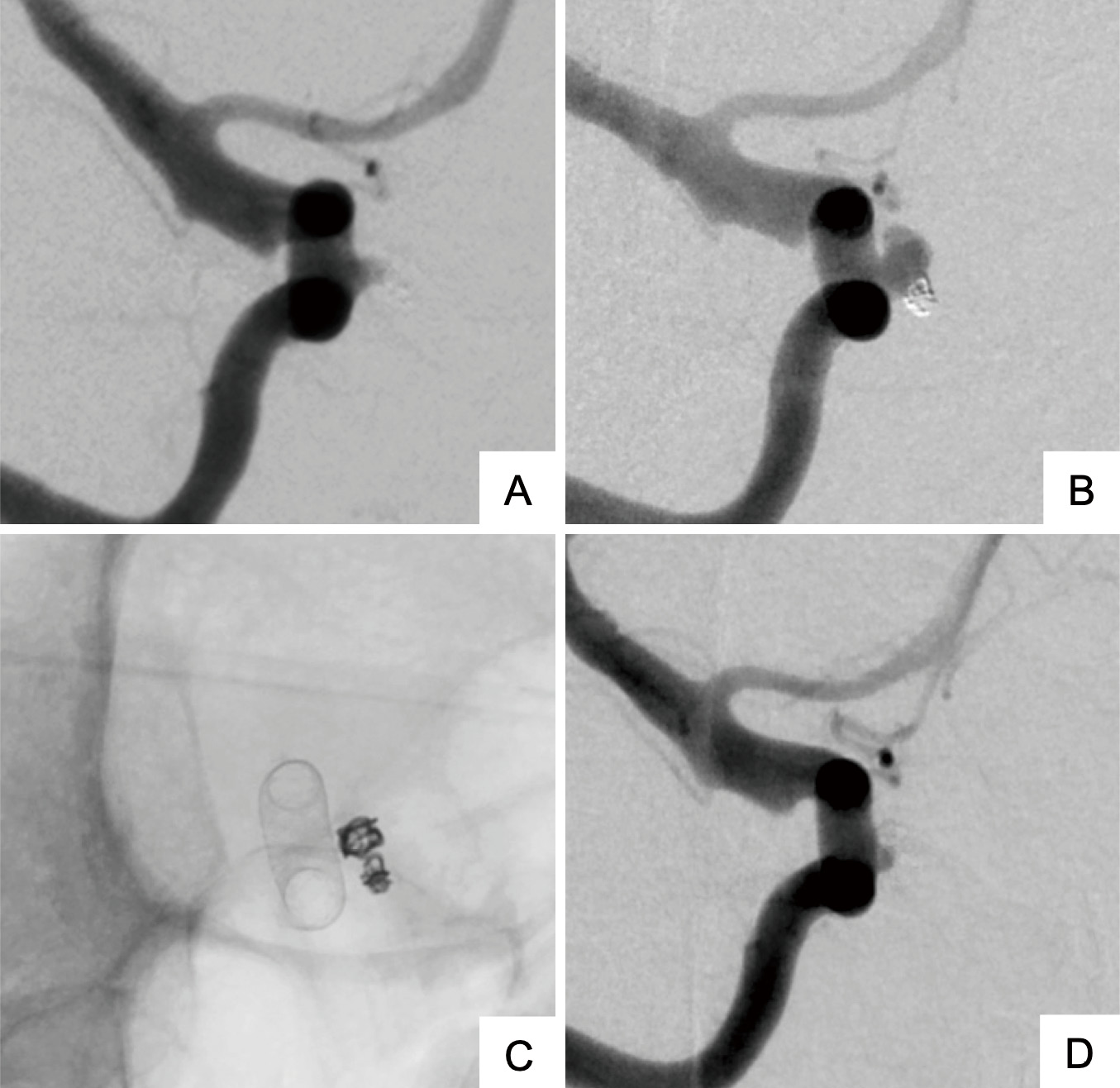
Digital subtraction angiography (DSA) images of endovascular treatment.
(A) DSA image following the initial coil embolization. (B) DSA image at the time of recurrence 40 months after the initial treatment. (C) DSA image immediately after flow diverter stent treatment. (D) DSA image 6 months following flow diverter device placement, showing almost a complete occlusion of the aneurysm.
Postoperatively, the patient was treated for vasospasm as per the usual treatment and was discharged without rebleeding or any neurological deficits. Although the patient had not shown symptoms, a further endocrine examination revealed diabetes mellitus and high prolactin levels (>2000 ng/mL); other abnormalities in blood hormone levels were not observed. However, we diagnosed the patient with asymptomatic prolactinoma because there was no loss of sexual desire or occurrence of gynecomastia. A follow-up MRI was performed at the outpatient department, and no medication was administered for prolactinoma due to concerns regarding side effects, such as aneurysm recurrence and CSF rhinorrhea. No noticeable tumor enlargement was observed; however, recurrence was suspected, based on the results of magnetic resonance angiography performed after 40 months. DSA revealed aneurysm regrowth with compaction of the coils inserted during the initial treatment (Fig. 2B). After obtaining sufficient informed consent, we decided to perform flow diverter device (FDD) placement.
After 2 weeks of preoperative dual antiplatelet therapy, a guiding catheter was placed into the right ICA via the femoral artery. A few platinum coils were placed into the aneurysm, following which a Pipeline Flex embolization device (Medtronic, Minneapolis, MN, USA) was deployed in the parent artery (Fig. 2C). No ischemic or hemorrhagic complications occurred postoperatively, and the patient was discharged as scheduled. DSA performed 6 months after FDD treatment showed almost complete occlusion of the aneurysm (Fig. 2D).
We made two crucial clinical discoveries in the present case report. First, although some cases of ruptured aneurysms following medical treatment for prolactinomas have been reported, even an untreated prolactinoma can cause bleeding from ICA aneurysms. Second, a reconstructive endovascular treatment might be an option for pseudoaneurysms.
Here, pseudoaneurysm was diagnosed as the rupture rate of small true nonbifurcating ICA aneurysms is generally slim, marked destruction of the sphenoidal bone by the pituitary tumor was found, and the tumor encased the aneurysm. Pseudoaneurysms can subsequently result in other complications, such as SAH, epistaxis, and direct carotid-cavernous fistula, which could lead to permanent neurological deficits or death. Patients with PA are known to have a higher likelihood of coexisting brain aneurysms, approximately seven times greater than other types of brain tumor patients, with occurrence rates ranging from 0.5% to 7.4%. Mercuri et al. reported a review of 150 cases of coexisting brain aneurysms and PA.10) The nonsecreting type was the most common, accounting for 43% of cases, followed by GH-producing tumors at 34%. The location of the tumors was mainly in the cavernous portion, representing 71% of the cases. Therefore, the factors contributing to aneurysm formation are believed to be influenced not only by secreted hormones but also by pressure and infiltration into the cavernous sinus. The rupture of an ICA-cavernous aneurysm is generally infrequent due to the protective nature of the surrounding rigid tissues such as the dura mater and bony structures. Nonetheless, the absence of such protection, brought on by the absence of surrounding tissue or the presence of a tumor mass, may heighten the likelihood of rupture.
We searched studies published from 1964 to 2021 on PubMed, using the following search terms: "prolactinoma" or "pituitary tumor;" "subarachnoid hemorrhage," "subarachnoid bleeding," or "epistaxis;" and "aneurysm." Cases of hemorrhage or aneurysm directly related to the surgical procedure under study and those with unknown details were excluded. Ruptured cases of true aneurysms coexisting with PAs but not in contact with the tumor were also excluded. Table 1 summarizes the details of this case and seven other similar cases.7-9,11-14) The cases are listed in order according to the publication year. All the pituitary tumors in the present review were prolactinomas, which caused an intracranial hemorrhage in six of eight cases. The present case was the only one in which an aneurysm occurred in an untreated prolactinoma, leading to concurrent SAH and epistaxis. There was another case of untreated prolactinoma with a poor prognosis due to massive epistaxis while waiting for surgery.11) In the remaining cases, the prognosis was relatively good if adequate treatment is completed, except for one case of sarcomatous transformation of a pituitary tumor.12) Aneurysms associated with giant prolactinomas are often partially or completely embedded within the tumor, similar to the present case, requiring an optimal treatment strategy tailored to the patient.10)
| Author (Year) | Patient age and sex | Clinical onset | Tumor characteristics | Treatment provided before rupture | Aneurysm location | Treatment for ruptured aneurysm | Outcome, FU |
|---|---|---|---|---|---|---|---|
| NA: not applicable; FU: follow-up; SAH: subarachnoid hemorrhage; DCCF: direct carotid-cavernous fistulae; TSS: transsphenoidal surgery; STA-MCA: superficial temporal artery-middle cerebral artery | |||||||
| Imamura et al. (1998)11) | 72Y, Female | Repeated epistaxis | Prolactinoma | Untreated | Cavernous portion | NA | Death (massive nasal bleeding) |
| Tachibana et al. (2000)12) | 21Y, Female | SAH | Sarcomatous transformation of prolactinoma | 3 TSSs, bromocriptine, and chemoradiation | Supraclinoid portion | NA | Death |
| Endo et al. (2011)13) | 62Y, Female | SAH and epistaxis | Prolactinoma | 2 TSSs and stereotactic radiosurgery | Petrous portion | STA-MCA bypass and internal trapping | Good, no FU |
| Akutsu et al. (2014)8) | 58Y, Female | SAH (immediately after a dural incision during a scheduled craniotomy) | Prolactinoma | Cabergoline therapy | Cavernous portion | STA-MCA bypass and proximal ligation | Good, no FU |
| Peng et al. (2015)14) | 53Y, Male | Epistaxis, pituitary apoplexy, and bilateral ptosis | Prolactinoma | (Intraoperative bleeding due to the absence of a preoperative diagnosis of aneurysm) | Cavernous portion | Proximal trapping | Good, 14 months |
| Khalsa et al. (2017)9) | 61Y, Male | SAH, right ptosis, and diplopia | Prolactinoma | Cabergoline therapy | Cavernous portion | Internal trapping | Good, 4 months |
| Nakahara et al. (2019)7) | 61Y, Female | Subdural hematoma and DCCF | Prolactinoma | Cabergoline therapy | Cavernous portion | 1) Coil embolization, 2) STA-MCA bypass and proximal ligation | Good, 2 months |
| Present case (2022) | 61Y, Male | SAH, epistaxis, and amaurosis | Prolactinoma | Untreated | Infraclinoidal portion | 1) Coil embolization, 2) flow diverter stent placement | Good, 43 months |
Medical treatment of asymptomatic prolactinomas has advantages and disadvantages. In the present case, because no symptoms of hyperprolactinemia were noted and the patient experienced simultaneous rhinorrhea and SAH, we intentionally did not attempt to treat the patient with medications due to concerns regarding the risk of CSF rhinorrhea and rebleeding following tumor shrinkage. After the aneurysm recurred, prolactin levels and other pituitary functions were measured; however, there were no changes. There has been no apparent increase in tumor volume, and the patient is followed up regularly in the 4 years. If there is an apparent trend of tumor growth, we will strategize a cabergoline therapy of low dosage while examining the aneurysm. In the case of giant prolactinomas, some opinions suggest that priority should be given to cabergoline treatment for tumor reduction and normalization of PRL level due to their high invasiveness and the fragility of the aneurysm wall.10) However, three cases of tumor shrinkage and aneurysm rupture following cabergoline treatment have previously been reported,7-9) suggesting that we should be aware of the possibility of aneurysm coexistence. In the presence of coexisting aneurysms, aneurysm treatment should be prioritized.
Since the recent introduction of microstents with high mesh density, there have been two main types of treatment for dissecting aneurysms and pseudoaneurysms: deconstruction and reconstruction. Deconstruction involves trapping or ligation with ICA sacrifice. Vascular sacrifice remains the most durable and reliable treatment for uncontrolled bleeding in the acute phase.3) By contrast, vascular preservation can be considered in situations of controlled bleeding or chronic recurrence. Meanwhile, some studies have reported the outcomes of reconstruction using FDDs and covered stents to preserve the patency of the mother vessel. Ghorbani et al. reviewed endovascular reconstruction for iatrogenic ICA injury following endonasal surgery. Both FDD and covered stents resulted in good clinical outcomes without significant differences.15) In another review of endovascular treatment for ICAPA following nasal surgery, Sylvester et al. reported that ICA sacrifice was performed in 40% of the cases, coil embolization in approximately 30%, and endoluminal reconstruction focusing on covered stents in 30%. Regarding reconstructions, covered stents were associated with technical complications in approximately 40% of the cases, suggesting that FDD is more secure.3) The present case showed an asymptomatic but noticeable regrowth and recurrence; FDD treatment was performed and showed good clinical outcomes.
In this report, we presented a rare case of a ruptured ICA aneurysm in an untreated prolactinoma, which resulted in SAH and epistaxis. Besides the conventional treatment of parent artery occlusion, reconstructive endovascular therapy may be a treatment option.
BTO: Balloon test occlusion
CSF: Cerebrospinal fluid
CT: Computed tomography
CTA: CT angiography
DSA: Digital subtraction angiography
FDD: Flow diverter device
ICA: Internal carotid artery
ICAPA: Internal carotid artery pseudoaneurysm
MRI: Magnetic resonance imaging
PA: Pituitary adenoma
SAH: Subarachnoid hemorrhage
The authors received no financial support for the research, authorship, and/or publication of this article.
The patient provided consent to the submission of this case report to the journal.
The authors declare no conflicts of interest.