2023 Volume 10 Pages 273-278
2023 Volume 10 Pages 273-278
Herein, we report a case of carotid artery stenting with proximal flow protection for severe stenosis of the left internal carotid artery using transbrachial and transradial artery approaches. Because an abdominal aortic aneurysm was present, we avoided the transfemoral approach. The procedure was successfully performed with a combination of an 8-Fr balloon guide catheter and microballoon catheter on separate axes. No complications such as pseudoaneurysm, thrombosis, or dissection were observed at the puncture site. The patient was discharged without complications and showed good outcomes at 3 months. This technique may offer a useful alternative for patients with severe stenosis who cannot be treated using a femoral artery approach.
The proximal protection (PP) method during carotid artery stenting (CAS) is useful for patients at high risk of distal embolization, such as those with severe internal carotid artery (ICA) stenosis or large amounts of unstable plaque.1) The transfemoral approach is commonly used for the PP method during CAS. However, advancing a guide catheter from the femoral artery to the carotid artery is difficult in patients with a tortuous aortic arch, supraaortic dissection, aortic disease, or unfavorable femoral artery occlusion. We report here a case of CAS with an alternative PP method using a balloon guide catheter (BGC) and microballoon catheter (MBC) in combination with transbrachial and transradial approaches to yield favorable outcomes.
A 67-year-old man presented to our hospital after severe stenosis of the left ICA was incidentally found during screening carotid ultrasonography performed to investigate mild memory dysfunction. His medical history included hypertension, dyslipidemia, and an abdominal aortic aneurysm 44 mm in diameter (Fig. 1A). The patient was also a current smoker, with a 40-year history of smoking one pack of cigarettes a day. The patient had a history of chronic obstructive pulmonary disease, and preoperative evaluation of spirometry function showed a decreased forced expiratory volume in 1 second of 51%. Magnetic resonance imaging (MRI) demonstrated no abnormalities such as old cerebral infarction or ischemic changes, but magnetic resonance angiography demonstrated a decreased signal from the left intracranial ICA due to severe stenosis (Fig. 1B). Carotid ultrasonography revealed a vulnerable plaque at the origin of the left ICA and an increase in peak systolic velocity to 400 cm/s. MRI of the neck plaque demonstrated high-intensity signals on time-of-flight imaging (Fig. 1C), magnetization-prepared rapid gradient echo imaging (Fig. 1D), and T1-weighted black blood imaging (Fig. 1E). On the basis of these findings, we diagnosed a vulnerable plaque with intraplaque hemorrhage. Left carotid angiography revealed anterograde string-like filling of the ICA beyond the carotid bifurcation (Fig. 1F) and showed that the stenosis had prolonged cerebral circulation time in the left cerebral hemisphere (Fig. 1G). A collateral pathway via the ipsilateral posterior communicating artery was also observed. Right brachial angiography showed no prominent coiling or bending of the brachial artery (Fig. 1H) and a well-developed deep brachial artery (Fig. 1I). Contrast-enhanced computed tomography angiography (CTA) revealed a bovine aortic arch (Fig. 1J). Vascular echocardiography showed that the right brachial artery at the cubital fossa had a vascular diameter of approximately 4.7 mm (Fig. 1K). Because of the problem of decreased respiratory function, we considered that carotid endarterectomy and direct carotid artery puncture requiring general anesthesia should be avoided because of the risk of postoperative respiratory complications. To address severe carotid stenosis, CAS was planned after obtaining an informed consent.
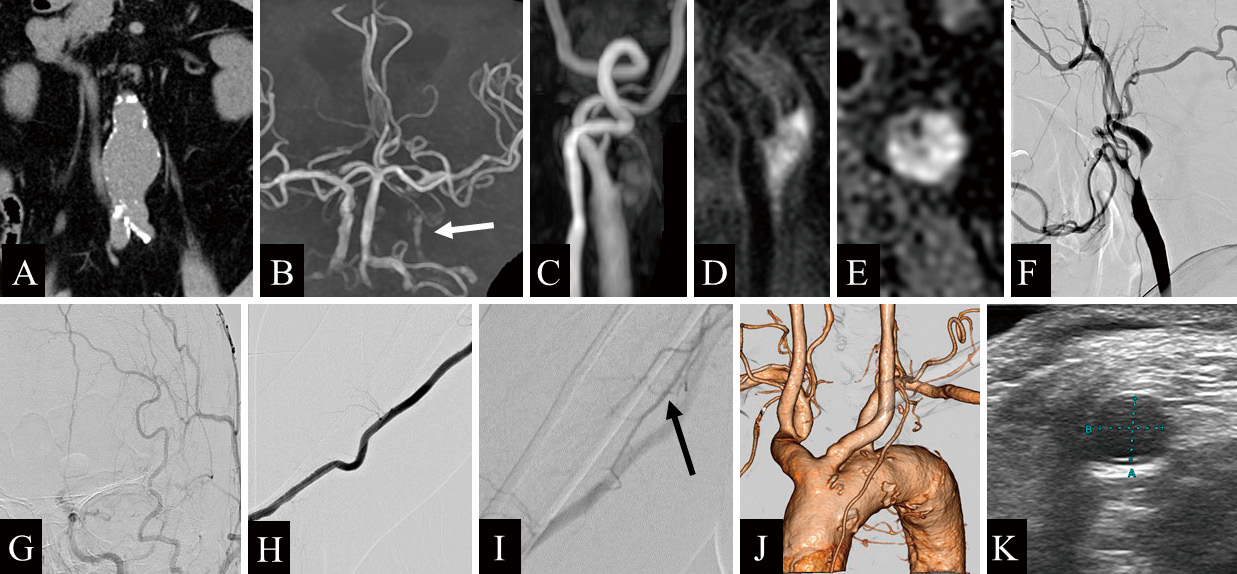
A) Computed tomography (CT) shows an abdominal aortic aneurysm, 44 mm in diameter. B) Magnetic resonance angiography of the head reveals a decreased signal from the left intracranial internal carotid artery (ICA) due to severe stenosis (white arrow). C) Time-of-flight magnetic resonance imaging demonstrates a high-intensity signal of the common carotid artery (CCA). D) Magnetization-prepared rapid gradient echo imaging shows a high-intensity signal in the CCA. E) T1-weighted black blood imaging reveals a high-intensity signal from the CCA, suggesting a vulnerable plaque. F) Left CCA angiography (lateral view) shows anterograde string-like filling of the ICA beyond the carotid bifurcation. G) Left CCA injection (frontal view) demonstrates prolonged cerebral circulation time in the left cerebral hemisphere. H) Contrast-enhanced CT angiography shows a bovine-type arch of the aorta. I, J) Right brachial angiography demonstrates a well-developed deep brachial artery (black arrow). K) Vascular echocardiography shows that the right brachial artery at the cubital fossa has a vascular diameter of approximately 4.7 mm.
Because of the large amount of plaque and near-occlusion, we considered that CAS using the PP method was desirable to adequately prevent distal embolization during the passage of the stenosis. However, due to the abdominal aortic aneurysm, the transfemoral artery approach was considered a high risk, so we decided to use an upper limb for the approach route. A PP device such as a Mo.Ma (Medtronic, Minneapolis, MN, USA) would have been desirable in this case but was avoided because of the high risk of puncture complications due to the need to puncture the brachial artery with an 8- or 9-Fr sheath. Furthermore, because two types of balloon are required to perform the PP method, we decided to perform CAS using a combined ipsilateral transbrachial and transradial approach wherein the BGC and MBC are guided from the brachial and radial arteries, respectively. An approach using the right and left upper limbs was also considered, but given the possibility of access difficulty due to the bovine-type bifurcation of the left common carotid artery (CCA), a tandem puncture was performed ipsilaterally instead.
The procedure was performed with the patient awake and under minimal sedation. Venipuncture was first performed through the right femoral vein, placing a 3-Fr sheath for arterial blood return. The right brachial artery was punctured with an 18-G needle, and a 0.035-inch-diameter guidewire was inserted into the outer casing to the subclavian artery. An 8-Fr BGC (OPTIMO; Tokai Medical Products, Aichi, Japan) with a dilator (TMP dilator I; Tokai Medical Products) was inserted into the lumen of the BGC and advanced into the right subclavian artery along the guidewire. After systemic heparinization, a 4-Fr sheath was inserted into the right radial artery, and a 4-Fr catheter (JB2 100 cm; Medikit, Tokyo, Japan) was carefully navigated into the left CCA (Fig. 2A, B). When advancing the 4-Fr catheter, no resistance attributable to interference with the BGC was encountered. Then, the 8-Fr BGC was navigated into the left CCA. The subsequent treatment procedure is shown in Fig. 3. A 0.014-inch microguidewire (CHIKAI; Asahi Intecc, Aichi, Japan) was placed deep into the external carotid artery (ECA), and the MBC (Pinnacle blue 20; Tokai Medical Products) was placed at the origin of the ECA along the microguidewire. Then the ECA and CCA were occluded with the BGC and MBC, forming a flow-reversal procedure. At that time, we confirmed the absence of side leakage aside from the gap between the BGC and shaft of the MBC. Using the roadmap technique, the 0.014-inch microguidewire was carefully advanced across the stenotic lesion while applying manual blood suction from the BGC, and dilation was performed using a 2.0 mm× 40 mm balloon catheter (Coyote; Boston Scientific, Natick, MA, USA) at the site of stenosis. The microguidewire was subsequently exchanged for a Spider Rx distal filter protection device (Medtronic/Covidien), which was positioned within the left distal ICA. Because the left superior thyroid artery bifurcated from the left CCA, the procedure with ECA and CCA occlusions was continued. Predilation was performed using a 4.0 mm× 40 mm balloon catheter (Sterling; Boston Scientific) at the site of stenosis. After that, an 8 mm× 30 mm carotid stent (CASPER Rx; MicroVention, Terumo, Tustin, CA, USA) was placed. Postdilation was performed using a 4.5 mm× 40 mm balloon catheter (Sterling; Boston Scientific) at the site of stenosis (Fig. 2C). After the left ECA and CCA balloons were deflated, all procedures were completed. The brachial artery was manually hemostatic for 20 minutes, followed by fixation with a hemostatic tape at a pressure of 60 mmHg for 6 hours. The radial artery was fixed with a band for 4 hours to achieve hemostasis. Left CCA injection showed no contrast-enhanced defect (Fig. 2D, E). No perioperative complications were observed, and ultrasonography showed no plaque protrusion. Contrast-enhanced CTA 5 days after CAS showed no abnormalities in the right brachial (Fig. 2F) and radial (Fig. 2G) arteries. Finally, the patient was discharged with a modified Rankin scale score of 0. On follow-up at 3 months after discharge, modified Rankin scale score remained 0. Consent for publishing this report was obtained from the patient.

Angiography during carotid artery stenting. A) An 8-Fr balloon guide catheter (BGC) is navigated from the right brachial artery (white arrowhead), and a 4-Fr catheter is guided from the right radial artery (white arrow). B) BGC and 4-Fr catheter navigated to the left common carotid artery (CCA). C) Fluoroscopic lateral view in which postdilation has been performed after placement of the carotid stent using a flow-reversal system with a microballoon catheter (MBC) (black arrow) and a BGC (black arrowhead). D) Left CCA injection (lateral view) after all procedures were completed. E) Left CCA angiography (frontal view) reveals no contrast-enhanced defect. F, G) Contrast-enhanced computed tomography angiography after 5 days of intervention shows no puncture site complications in the right brachial (F) and radial (G) arteries.

Schematic illustration of treatment procedures during carotid artery stenting. A) After placement of the balloon guide catheter (BGC) and a 4-Fr catheter. B) The microguidewire (MG) is inserted deep into the external carotid artery (ECA), with the microballoon catheter (MBC) placed at the origin of the ECA alongside the MG. C) The ECA and common carotid artery are occluded with the BGC and MBC, forming a flow-reversed system. The MG is advanced across the stenotic lesion. D) The MG is exchanged for a filter protection (FP) device after angioplasty with the balloon catheter (BC). Predilation and postdilation with BC and placement of a carotid stent (CS) are performed. E) The MBC is deflated and removed first; then, the BGC is deflated. All procedures are completed.
Endovascular treatment via a brachial or radial approach has been increasing in recent years, and CAS is no exception. This case was novel in that CAS was performed using PP method via the brachial and radial arteries. The key points of this case can be divided into PP via transbrachial and transradial artery approaches and points to keep in mind when performing this procedure, along with a discussion of the literature.
PP method via the transbrachial and transradial arteriesIn this case, the transfemoral approach was avoided because of the presence of an abdominal aortic aneurysm, and the transfemoral approach is commonly used when CAS is performed using a PP method.2) Previous studies have shown that CAS using the PP method has a low risk of perioperative cerebral infarction.1,3) This is achieved using a dedicated PP device such as Mo.Ma or GORE (Gore & Associates, Flagstaff, AZ, USA), but the transbrachial approach is rarely used because of problems with device diameter, stiffness, and arterial bifurcation morphology. As of 2022, the PercuSurge Guardwire (Medtronic) is no longer available, so the Parodi method, which uses the BGC and PercuSurge Guardwire in combination to block the ECA and CCA, cannot be performed.4) In this method, a PercuSurge Guardwire was placed coaxially in the BGC and implanted in the ECA to create a flow-reversal circuit; however, currently, no device with a balloon can be inserted coaxially when performing CAS. Therefore, we performed CAS using the PP method with the BGC and MBC on separate axes, using both transbrachial and transradial approaches.
Relatively few reports in the literature on CAS have described using a transbrachial or transradial approach with the PP method. Montrsi et al.5) prospectively reviewed the outcomes and complications of CAS performed using transbrachial or transradial approaches. They inserted an 8-Fr sheath into the brachial or radial artery and performed 61 CAS procedures under PP method using an 8-Fr Mo.Ma. The mean stenosis rate was 87.8% in the European Carotid Surgery Trial.6) The brachial artery was used in 31 cases (51%) and the radial artery in 30 cases (49%), and the procedure was completed in 55 cases (90%). Of the six patients for whom the procedure could not be completed, Mo.Ma could not be induced because of anatomical reasons in one patient (1.6%), and the blockade was not tolerated by five patients (8.1%). Complications included pseudoaneurysm of the brachial artery in one case (1.6%) and acute occlusion of the radial artery after surgery in two of 30 cases (6.6%). The present report is rare in that the 8-Fr sheath was inserted not only via a transbrachial approach but also via a transradial approach, and the procedure was performed using Mo.Ma.
Points when performing this procedureThe following is a list of points to be considered in this procedure. First, the diameter of the brachial artery is important. In this procedure, two catheters are navigated from the same upper extremity through the brachial artery. For example, the outer diameter of an 8-Fr BGC is approximately 2.7 mm and that of a 4-Fr catheter is approximately 1.3 mm, so the brachial artery must have an inner diameter of ≥4.0 mm. In this case, the diameter of the brachial artery was measured preoperatively using echocardiography and confirmed as 4.7 mm, and thus, the procedure was deemed feasible. The diameter of the brachial artery is estimated to be approximately 4.5 and 3.5 mm in adult men and women, respectively.7,8) The order of puncture is also important, and the brachial artery should be punctured first. If the radial artery is punctured first to guide the device, the puncture of the brachial artery may damage the device. To reduce puncture site complications as much as possible, BGC was inserted using a 5-Fr long dilator into the brachial artery without a sheath. Koge et al.9) reported a case in which a BGC was safely guided into the CCA without the need for a sheath, using a dedicated 6-Fr long dilator in combination with a 9-Fr BGC. However, in the cardiovascular field, bleeding complications at the puncture site were observed in 2.3% of patients using the transbrachial approach,10) which is higher than the rate of 2.0%10) seen when using the transfemoral approach. Furthermore, 6-Fr intra-aortic balloon pumping has reportedly shown fewer puncture site complications than 8-Fr IABP.10) Another report found that the insertion of a sheath with a diameter larger than 8-Fr was associated with pseudoaneurysm as a complication at the puncture site.11) In other words, the brachial artery approach using an 8-Fr sheath is considered a high risk in Japanese patients who have smaller arterial diameters than Westerners. We used a sheathless technique with an 8-Fr BGC to reduce the size of the puncture site to that of a 6-Fr sheath. However, the risk of puncture site complications in the upper extremities is expected to increase due to ipsilateral radial artery puncture in addition to brachial artery puncture. It might have been better to remove the 4-Fr catheter after dilating the MBC to reduce the risk of upper extremity ischemia. This procedure is indicated only for very specific cases with large brachial arteries and is not recommended for universal use in Japanese patients, who, as already mentioned, have smaller arterial diameters in the upper extremities than Westerners.
The order of hemostasis may also be important. In our case, hemostasis was applied to the brachial artery, which has a large volume of perfusion to the upper limb, followed by hemostasis of the radial artery. However, if hemostasis was applied to the brachial artery while a sheath was inserted into the radial artery, perfusion to the ulnar artery, which is a collateral blood vessel, would be reduced, possibly resulting in further hypoperfusion inhibition. The collateral vessels function in the deep brachial artery12) when the brachial artery is occluded and in the interosseous artery when the radial and ulnar arteries are occluded. Therefore, evaluating this information preoperatively would be desirable.
Difficulty in guiding the device should also be noted. In particular, in left-sided lesions with a normally shaped aortic arch (e.g., arch type I), the angle of bifurcation of the CCA is so steep that the device may vector toward the ascending aorta and slide off during device advancement. In the present case, the device was easily guided to the left CCA because the aortic arch was the bovine type. However, an MBC could conceivably be guided from the left upper extremity using a Simmons-type guiding catheter.
With the method we applied, the MBC is guided in parallel with the BGC and implanted at the origin of the ECA, rather than coaxially with the BGC. Therefore, when the balloon of the BGC is inflated, a gap exists between the shaft of the MBC and BGC, which may prevent complete blockage of progressive blood flow. In this case, we used a contrast medium to check for residual progressive blood flow after the blood flow was supposed to have been blocked. Fortunately, we confirmed that complete blood flow blockage had been achieved. However, depending on the degree of balloon expansion of the guiding catheter, leakage or damage to the vessel wall may occur. Caution is therefore warranted. Another attractive point is the ease of balloon positioning: the distance between ECA and CCA balloons cannot be changed in Mo.Ma.
This presents a review of the literature. This technique may be useful in CAS for ICA stenosis with severe stenosis where intraoperative distal embolization is a concern, when a conventional transfemoral approach is difficult, and when anatomical access is considered possible.
We have described a case of CAS with the PP method for severe left ICA stenosis using a combination of transbrachial and transradial artery approaches. This method may be effective in cases of severe stenosis with a high risk of distal embolization and anatomical abnormalities preventing the use of the femoral artery approach.
All authors have no conflicts of interest.