2018 Volume 94 Issue 1 Pages 20-34
2018 Volume 94 Issue 1 Pages 20-34
Immediately after the Fukushima nuclear power plant accident, a team of 40–50 researchers at the Graduate School of Agricultural and Life Sciences at the University of Tokyo began to analyze the behavior of radioactive materials in the fallout regions. The fallout has remained in situ and become strongly adsorbed within the soil over time. 137Cs was found to bind strongly to the fine clay, weathered biotite, and organic matter in the soil; therefore, it has not mobilized from mountainous regions, even after heavy rainfall. In farmland, the quantity of 137Cs in the soil absorbed by crop plants was small. The downward migration of 137Cs in soil is now estimated at 1–2 mm/year. The intake of 137Cs by trees occurred through the bark and not from the roots. This report summarizes the findings of research across a wide variety of agricultural specialties.
Over six years have passed since the Fukushima nuclear plant accident (FNPA) that occurred following the Great East Japan Earthquake on March 11, 2011. The total radioactivity released into the environment was estimated at 770,000 teraBq, or approximately 15% of that released by the Chernobyl accident. Initially, the radioactive nuclides released by FNPA contained 21% 131I (half-life: 8 days), 2.3% 134Cs (half-life: 2 years) and 1.9% 137Cs (half-life: 30 years). Now, those remaining in the environment consist mostly of 134Cs and 137Cs, the ratio of which has changed from roughly 1:1 in 2011 to 0.12:1 in 2017.
The radioactive fallout was mostly distributed in Fukushima prefecture (Fig. 1), where extensive attempts to remove the fallout have been conducted. To secure the safety of agricultural production, for example, the government has promoted the application of K fertilizer to fields to prevent uptake of radioactive Cs into crops. The prefecture office has taken charge of inspecting all agricultural products for radioactivity before they enter the market. In the case of rice, more than 10,000,000 bags (30 kg rice/bag) have been monitored every year. Contaminated bags, with a radioactivity of higher than 100 Bq/kg, decreased from 71 bags in 2012 to zero in 2015. All agricultural products in the market are now controlled below the threshold levels for radiation exposure.1)–4)

Radiation dose rate after FNPA (Apr. 29, 2011). http://ramap.jmc.or.jp/map/eng/.
However, to promote the further recovery of agriculture, characterization of the radioactive contamination in the integrated agricultural environment, including cultivated fields, forests, mountains, and water systems, is essential. A voluntary team consisting of 40–50 academic faculty members from the Graduate School of Agricultural and Life Sciences at the University of Tokyo, all specialists on soil, crops, wild and domestic animals, fishery, forestry, and other fields, came together immediately after the accident.5),6) They repeatedly entered contaminated areas to conduct collaborative research on various aspects of the contamination. The following review summarizes the achievements of this multidisciplinary approach.
Because the accident occurred in March, most of the agricultural land was in a fallow state after the previous year’s harvest, except for that used by wheat crops. Most of the fallout was directly scattered on the exposed surface of the soil and forest litter at the time of the accident. Radiography shows the spotted distribution of radioactivity on the surface of soil and litter (Fig. 2A–C).5),7) Successive monitoring revealed that 134Cs and 137Cs remained at the initial points of deposition without moving; therefore, these radionuclides would be difficult to remove from the fields. Figure 2D–E shows the results from radiographic analyses of wheat with a developed ear harvested two months after the accident. Most of the radioactivity was observed in the oldest leaves, which were exposed to the air at the time of the accident. This result shows that the fallout had stayed at the initial attachment points on the leaf surface and had not been removed. When the radiographs of the wheat leaves were magnified, the distribution of the contamination was still spotlike, indicating no incorporation of 134Cs and 137Cs into the phloem or xylem systems of the leaves. Because the amount of fallout incorporated into the plant was very small, the radioactivity of new leaves was very low. Additionally, the radioactivity of the ear was 2,000 times lower than that of the oldest leaf.8),9)

Spotlike radioactive fallout on soil, wheat, forest litter, and bird feathers. The overall feature of the fallout was that the radiocesium remained at the initial contact site and did not subsequently move. A: 15 g of soil was collected, and an autoradiograph was taken with an imaging plate. Bar: 10.0 mm. B: Forest litter collected in the mountains. C: Autoradiograph of the collected litter shown in B. D: Wheat plant harvested two months after the accident when ears were developed. E: Autoradiograph of the harvested wheat shown in D. F: Tail feather of the male bush warbler. G: Autoradiograph of the tail feather shown in G.6),7)
In forested areas, deciduous trees did not have leaves at the time of the accident, but the needle-like leaves of evergreen trees adsorbed high amounts of radioactive fallout. The soil under these trees was thus less contaminated by radioactivity. The leaves of evergreen trees located higher in the crown were also more contaminated than those located lower.10)
Spotlike radioactive contamination was also found on the feathers of birds, such as the male bush warbler, captured in a highly contaminated area close to the nuclear plant in 2011 (Fig. 2F, G). This contamination was not removed by washing, but in the following year, no radioactivity was found in the feathers of the same bird species, probably due to molting.11)–13)
The main feature of the pedological profile of the soil in Fukushima prefecture’s paddy fields is Gray Lowland soil (51%), Gley soil (17%), and Andosol (10%), compared to the national profile of 36% Gray Lowland, 30% Gley, and 10% Andosol. In upland fields, the percentage of Brown Forest soil is 2.2 times higher, and the Andosol proportion is approximately 60% lower, compared to the average values in Japan. However, the extent of contamination was similar in both paddy and upland fields. In 2011, highly contaminated areas containing more than 5000 Bq/kg of 137Cs in the soil represented approximately 6% of the land area of Fukushima prefecture, while less contaminated areas with less than 100 Bq/kg of 137Cs represented greater than 57% of the land area.
2.1. Vertical migration of radioactivity in soil.Approximately two months after the accident, on May 24, 2011, a vertical gradient of 134Cs and 137Cs in the top 0–15 cm of soil was measured in an undisturbed paddy field. Roughly 20 g of soil was taken from different depths and placed into a 20-ml vial, and the radioactivity was measured using a Na(Tl)I counter (2480 WIZARD, Perkin Elmer) for 20–30 min. The sample was then transferred to a U8 vessel, and the radioactivity of 134Cs and 137Cs was measured using a Ge counter (GEM type, SEIKO EG&G). The results showed that 96% of 134Cs and 137Cs was present in the 0–5 cm layer, with the remainder in the 10–15 cm layer (Fig. 3C).5)

Fallout distribution in soil. Vertical concentration of radiocesium (134Cs and 137Cs) in the top 0–15 cm layer of soil was measured in an undisturbed paddy field. A: Scintillation counter covered with a lead collimator with a slit window. B: A vinyl chloride cylinder (0.6–1.3 m × 85 mmφ) was placed in a borehole made in the soil. C: 134Cs and 137Cs distribution along the depth. D: Autoradiograph of the soil separated according to the particle size. 1: surface soil, 2: organic matter, 3: silt, sand, and gravel, 4: clay and organic matter. Bar: 10.0 mm.15)
To measure the vertical distribution profile of 134Cs and 137Cs in the soil, a vinyl chloride pipe (0.6–1.3 m × 85 mmφ) was placed in a borehole in the soil. A scintillation counter, covered with a lead collimator with a slit window, was then inserted downward into the pipe to measure the radioactivity along the borehole (Fig. 3A, B). This measurement was repeated every few months to measure the downward movement of 134Cs and 137Cs. During the first three months after the accident, the movement of 134Cs and 137Cs in the soil was 21.6 mm. However, the mobility of 134Cs and 137Cs was reduced drastically to 5.6 mm during the next three months, even though the rainfall was more than three times higher.14),15) The downward movement of 134Cs and 137Cs is currently 1–2 mm/year (S. Shiozawa, personal communication).
When contaminated soil was washed just once with individual chemicals (e.g., potassium iodide, cesium iodide, calcium hydroxide, etc.) to extract radioactive cesium, approximately 20% of the radioactivity was extracted; further washing failed to remove the remaining radioactivity.16)
2.2. Soil materials adsorbing 137Cs.To obtain information about the materials adsorbing 134Cs and 137Cs in the soil, soil samples were separated according to particle size, and an autoradiograph was taken for each fraction (Fig. 3a–d).5) The results showed that the radiocesium was adsorbed by the fine clay and organic matter but not by larger components such as gravel or sand. This finding proved crucial to developing an efficient and practical decontamination protocol for farmland.
The next question was what kind of clay mineral adsorbed 134Cs and 137Cs. Eight mineral species of clays roughly 50 µm in size were prepared, and adsorption/desorption experiments were conducted using a small quantity of 137Cs tracer (0.185–1.85 Bq). Weathered biotite (WB), i.e., partially vermiculitized biotite, adsorbed 137Cs far more tightly than did the other clay minerals (fresh biotite, illite, smectite, kaolinite, halloysite, allophane, and imogolite). When the carrier-free 137Cs solution was mixed with WB, the adsorbed amount of 137Cs continued to increase for over a week without showing any saturation. This result differed from the adsorption curves of the other minerals, which showed saturation. When the desorption of 137Cs from these clay minerals was examined, the desorption from WB was found to be especially small.17)
To measure the distribution of 137Cs activity in a platy-shaped WB, a focused ion beam was used to cut the WB piece into four fragments. One of these fragments was further divided into four, and the radioactivity of these fragments was measured with an imaging plate. Each piece of WB showed similar radioactivity per volume. When the outer part of the specimen was removed, the radioactivity was reduced proportionally to the eliminated volume, suggesting a uniform distribution of 134Cs and 137Cs within WB (Fig. 4). This finding completely changed the understanding of the adsorption sites of clay minerals. The layered shape of clay has been reported to have a loose edge due to weathering, known as a frayed edge site. Radiocesium was originally expected to be fixed at the frayed edge site, but 137Cs distribution was uniform.18)

Radiocesium distribution in a clay particle. Weathered biotite (WB) was cut into four pieces, A to D, and fraction B was further cut into four pieces, b-1 to b-4, using a focused ion beam. The radioactivity of each piece was then measured with an imaging plate. Each separated fraction of the WB showed similar radioactivity per amount, suggesting the uniform distribution of radiocesium within the clay piece. (Ref. 18 revised).
Soils usually contain the stable nuclide of cesium (i.e., 133Cs). To clarify the differences in behavior between radiocesium and stable cesium, an agricultural area of 30 m × 3.6 m at Komiya field in the village of Iitate was selected in September 2014. In this area, the total 134Cs and 137Cs activity was 5000 Bq/kg, which corresponded to approximately 10−6 mg/kg, whereas the concentration of stable 133Cs was approximately 7 mg/kg. The area was divided into 60 sections, and the total amount of 137Cs and 133Cs, as well as the exchangeable amounts of 137Cs and 133Cs extracted with ammonium acetate, were measured in each section.
The exchangeable amounts of both radioactive and stable cesium were well correlated with the amounts of carbon (C) in the soil, suggesting that most of the exchangeable Cs was adsorbed to organic matter and that this organic matter concentration determined the amount of exchangeable Cs. In this field, the distribution of both exchangeable 137Cs and 133Cs showed similar patterns (T. Kogure, personal communication).
However, when the ratios of exchangeable cesium to total cesium were calculated, the ratio of exchangeable 137Cs to total 137Cs was roughly 2.5 times higher than that of exchangeable 133Cs to total 133Cs. Because this ratio should be the same when equilibrium is attained, the higher extraction ratio of exchangeable 137Cs suggests that the fixation of radiocesium to the soil is still proceeding. Exchangeable Cs is easily taken up by plants (N. Nihei, personal communication).
To study the transfer of 137Cs from soil to rice, the relationship between radioactivity in the soil and that in plants was measured. A reciprocal correlation was usually observed between the soil K concentration and the 137Cs concentration taken up by plants, suggesting that applying K to the soil prevents 137Cs uptake into the rice grain. Notably, when an optimum amount of K was not supplied to K-deficient fields, 134Cs and 137Cs content in rice plants was high.
Occasionally, all wheat plants could not be removed from a field, resulting in these plants being ploughed into the soil. Ploughing this contaminated crop residue into the soil for the subsequent planting of rice effectively reduced later 134Cs and 137Cs uptake by rice plants. Additionally, in the case of the vegetable komatsuna, ploughing the contaminated crop residue into the soil reduced 134Cs and 137Cs contents to less than half compared to those without ploughing. The cause of the observed decrease in 134Cs and 137Cs in plants remains unknown; nevertheless, this process was confirmed to effectively reduce the absorption of radiocesium.19)
3.2. Unusual rice contamination.Because the application of a relatively high amount of K to the soil was found to reduce the uptake of cesium in rice plants, the contamination of rice grain is now avoidable. However, in the city of Nihonmatsu in 2011, one case of rice grain with a contamination level exceeding the initial regulation value of 500 Bq/kg was observed; no K had been applied to the field. This field was unusual because it contained a relatively small amount of clay in the soil. In the past, weathered granite had been taken from the mountains to prepare the field for rice production. In addition, the permeability of water to deeper layers was low in this area; therefore, the soil always contained excess water, resulting in lower organic matter decomposition. These conditions are assumed to reduce 134Cs and 137Cs absorption by the soil and enhance the uptake of these radionuclides in rice plants.
Soon after this contamination was detected, all rice plants in this field were left in situ to prevent the contaminated rice from entering the food chain. The radiograph of one plant from the Nihonmatsu field is shown in Fig. 5A, B. The distribution of 134Cs and 137Cs within the plant was very unusual because the younger leaves accumulated higher amounts of radiocesium than did the older leaves, which was the reverse of how radiocesium accumulated in plants harvested in other areas of Fukushima prefecture. In addition, among the tissues of the sample plant, the two culms that developed just before the development of the ear showed the highest concentration of 137Cs (Fig. 5B). Because one culm requires approximately two weeks to develop, the observed 137Cs distribution indicated that some event stimulated the incorporation of radiocesium approximately four weeks before ear development.20),21)

Radioactivity distribution in a rice plant exceeding the initial regulation value of 500 Bq/kg. A: Rice plant and soil. B: Autoradiograph of A taken using an imaging plate.21)
Because of this unusual case, further field work was conducted to better understand why contaminated rice was produced. Although the application of sufficient K to the soil reduces the uptake of 137Cs in plants, other exceptional cases have been observed in which sufficient soil K failed to reduce radiocesium content in plants. A great deal of water is needed to grow rice; water is occasionally directly introduced to the rice field from rivers or a water reservoir specially prepared for rice cultivation. In one of these exceptional cases, water was taken from a reservoir in which algal colloidal particles were observed during the summer season; this water also contained high amounts of 134Cs and 137Cs. When this water was supplied, the 134Cs and 137Cs concentration increased in the plants. The radioactivity in the reservoir water was lowered by approximately 20% after filtration through a 0.7-µm glass filter (data not shown). Therefore, the enhanced uptake of radiocesium by rice plants likely occurred via previous accumulation in microalgae.
3.3. Laboratory work to confirm the effect of K.Laboratory work has begun to confirm how K supply reduces radiocesium uptake by plants. First, the accumulation of Na, Mg, Ca, K, and Cs in plant tissue was studied under K-deficient and K-optimum conditions. Rice plants were grown in water culture until the plants produced rice grains. The plants were then harvested, and the concentrations of Na, Mg, Ca, and K in each tissue were measured by digesting the tissue with nitric acid, followed by analysis using inductively coupled plasma-optical emission spectrometry (ICP-OES). The radioactivity of 137Cs was determined by gamma counting prior to the measurement of the elements. Under the K-deficient condition, K accumulated in the tissues located higher than the culm (i.e., the culm, husk, and brown rice) (Fig. 6). The amount of 137Cs in the ears was approximately 6–7 fold higher under the K-deficient condition compared to when sufficient K was supplied. Because the total amount of 137Cs in the aboveground part of the plant increased only 3 times under the K-deficient condition, this result indicates specific accumulation of 137Cs in the ear. Among the other elements, Na tended to be taken up to compensate for K deficiency, but no specific accumulation of Na was observed in the grain. The accumulation of both Mg and Ca was not highly influenced by K deficiency, suggesting a different translocation mechanism.22),23)

Concentrations of K, 137Cs, Mg, Ca, and Na in the tissues of K-sufficient (white bar) and K-deficient (black bar) rice plants at the harvesting stage. The concentrations of K, Mg, Ca, and Na in the tissues were measured using ICP-OES.21)
Regardless of the K condition, more than 95% of Cs in the ear was transferred from the accumulated 137Cs in the plant before the development of the ear. Therefore, K supply after ear emergence could limit 137Cs accumulation in the ear only to some extent.24) K starvation not only increased Cs content in the aboveground part of the plant but also induced the intensive transport of Cs to the younger leaves.23) Competition between Cs and K for uptake by plants was observed, even though the natural abundance of Cs compared to K is 1000-fold lower. Previous literature has discussed how the K transporter occasionally shows permeability to Cs.24),25) However, little is known about the movement of Cs within the aboveground parts, which has been examined mainly using the ratio of K to Cs in different tissues leading to the different Cs transporters. However, this ratio was dependent on the amount of K supplied.22)
A direct comparison of stable K and stable Cs behaviors in rice plants was conducted for the first time using double tracers, 42K and 137Cs. Although the uptake rate of K+ was 7–11 times higher than that of Cs+, this difference was not reflected directly in the concentration of both ions in total plant tissues. After entering from the root, the concentration ratio of 42K+ to 137Cs+ in the aboveground parts of the plant, including leaves, was approximately 4 times higher than that in the roots, indicating that there are different transporters for both ions in different tissues.26)
3.4. Radiocesium contamination of rice.Because the grain of boiled rice is the staple food in Japan, the distribution of radiocesium in the grain was investigated in detail. When rice grain was milled to remove the husk and bran layers, the radioactivity concentration was reduced by approximately 50%. By washing this milled grain, the radioactivity concentration was further reduced by approximately half.5) To prepare rice, water is added to the grain and steamed; this process again reduced the radioactivity concentration by approximately half. Therefore, the concentration of 134Cs and 137Cs in the rice was reduced to approximately 1/8 of that in the harvested rice grain when prepared for human consumption.
During the developmental stage of the rice, from approximately 10 days after flowering, the rice grain was periodically harvested. The grain was sliced and placed on an imaging plate to obtain radiographs. Radiocesium began to accumulate both in the hull and cereal germ of the grain as the ripening process proceeded. To study the distribution of radioactive Cs in greater detail, a microautography method was employed that had previously been developed in our laboratory.27) After the grain was sliced, a film emulsion was painted on the surface of the glass to produce a thin film. The film was exposed to radiation from the sample and then developed to obtain a microradiograph. When the microradiograph of the grain was examined under a microscope, 137Cs was found to have accumulated around the plumule and radicle, suggesting that 137Cs was not incorporated into the newly developing tissue itself but instead accumulated in the surrounding meristem tissue. This result was similar to the phenomenon that the meristem is generally protected and free from contamination of heavy metals or viruses (Fig. 7).28)

137Cs distribution in a rice grain. A: A rice grain was sliced, and the 137Cs distribution image was taken using an imaging plate. B: Microradiography of the rice grain prepared using nuclear emulsion film in contact with the sliced section. The upper left shows the image under a microscope, and the upper right shows the microradiograph. 137Cs concentration was not high in the newly developing tissue itself (i.e., plumule and radicle) but was higher in the surrounding tissue.28)
The accumulation of 134Cs and 137Cs in soybean seed tends to be higher than that in rice grain.2) One reason is that a soybean seed does not have an albumen. In the case of rice grain, 134Cs and 137Cs accumulated in the embryo and not in the albumen. The soybean seed itself develops into a cotyledon, a kind of embryo; therefore, it has a high mineral content. For example, the K and Ca contents in soybean seed are 7.6 and 24 times higher than those in rice grain, respectively, which could be the reason for the high accumulation of 134Cs and 137Cs. Another difference exists in how 134Cs and 137Cs accumulate in rice and soybean plants. In rice plants, 134Cs and 137Cs absorption occurs before ear emergence, and out of the total 134Cs and 137Cs absorbed, 10%–20% is accumulated in the grains. However, in soybean plants, half of the 134Cs and 137Cs accumulated in the seed is taken up during the period of pod formation, and approximately 42% of the total 134Cs and 137Cs absorbed accumulates in the seeds (data not shown).
3.6. Real-time Cs uptake imaging.A real-time radioisotope imaging system has been developed in our laboratory through which the movement of radioisotopes can be monitored, not only from the environment to the roots but also from the roots to the aboveground parts of a plant.29)–31) Using this real-time radioisotope imaging system, a discrepancy was observed between the radiocesium absorption of plants growing in water culture and that of plants growing in soil culture. In water culture, plants absorbed high amounts of 137Cs within hours, whereas in the paddy soil, 137Cs was trapped firmly by the soil and was unavailable for absorption by the plants (Fig. 8).28) In water culture, 137Cs dissolves as an ion and is thus easy for plants to absorb. However, in soil culture, the plant roots were unable to dissolve the 137Cs adsorbed to the soil, which resulted in less 137Cs absorption. Although water culture is a popular tool in the laboratory, it should be noted that water culture provides different results than those of soil culture and that field study is principally based on soil culture.

Radiocesium uptake in rice plants imaged by real-time radioisotope imaging. Upper: Principle of the real-time radioisotope imaging system. Lower: 137Cs uptake images after 2 and 18 hours of 137Cs supply. The left two plants were grown in water culture, and the right two plants were grown in soil. When rice plants were grown in soil containing 137Cs, the 137Cs was not absorbed by the plant because 137Cs was strongly adsorbed to the soil. However, in water culture, the plants absorbed the high amount of 137Cs dissolved in the culture solution.28)
In forested land, most of the radioactive material was trapped in leaves located high in evergreen trees, as well as in their bark; therefore, radioactivity was relatively low at the soil surface. During the past few years, the contaminated evergreen leaves have fallen to the ground, and as the litter has decomposed, 134Cs and 137Cs have gradually moved into the soil to be firmly adsorbed by soil particles.
The distribution of radioactivity was measured in a whole Japanese cedar tree (Cryptomeria japonica), downed in the city of Minamisoma in 2012. When radiographs were taken of wood disks from different heights, high radioactivity was found at the trunk surfaces of the disks. In higher sections, a considerable amount of radioactivity was also detected in the heartwood (Fig. 9).10),32) Although the amount of radioactivity that moved into the heartwood differed along the height of the trunk, the contamination inside the tree was not caused by the transportation of 134Cs and 137Cs from the roots. The active part of the roots for most trees is at least 20–30 cm below the surface of the soil, and no radiocesium is present at this depth, which suggests that the roots could not absorb 134Cs and 137Cs.

Radiocesium distribution within a cedar tree (Cryptomeria japonica). A cedar tree was cut down, and autoradiographs were taken of disks representing different heights of the tree.10)
Because timber logs used in the cultivation of mushrooms represent approximately 50% of the value of all forestry products in Japan, the reduction of radioactivity in mushrooms and timber are very important issues.33) The radioactivity contamination of mushrooms was examined in samples obtained from the experimental forests of the University of Tokyo throughout Japan. Several kinds of mushrooms were periodically harvested, and their radioactivity was measured. The radioactivity of these mushrooms has not only decreased drastically with time, but some mushrooms harvested more than 300 km from the site of the FNPA contained only 137Cs and not 134Cs, indicating that they are still accumulating the global fallout from nuclear weapons testing during the 1960s.34) Because the half-life of 137Cs (30 years) is much longer than that of 134Cs (2 years), all of the 134Cs in the fallout from the 1960s has decayed during the past 50 years.
The river water flowing from the mountains showed very low radioactivity (less than 10 Bq/l), and the water itself had even lower radioactivity after glass filter filtration. From the total fallout of 134Cs and 137Cs in the mountainous regions, approximately 0.1% per year was estimated to flow out via rivers.35),36)
In fruit trees, 134Cs and 137Cs moved from the surface of the bark into the wood, similar to the pattern observed for forest trees. This kind of radiocesium movement was first observed in orchards at an experimental farm in Tokyo where part of the soil surface was covered with a cloth before the accident. Because the radioactivity of the soil surface in the uncovered area was higher than that of the covered area, higher fruit contamination was expected in the trees grown in the uncovered area. However, the radioactivity of the harvested fruit was approximately equal between both sets of trees, indicating that 134Cs and 137Cs were not absorbed by the roots. The concentration of 134Cs and 137Cs was very high at the thin surface of the trunk, while the concentration was drastically reduced within the tree. However, when the total amount of 134Cs and 137Cs was calculated, the amount of both nuclides inside the tree was roughly the same as that of the surface skin, because the total mass of the inner tree was much higher than that of the skin surface (Fig. 10).37)

Radiocesium distribution in the trunk of a peach tree. Radioactivity inside the trunk. The radioactivity concentration (Bq/kg DW) was highest at the surface and gradually decreased toward the center (numbers below the white bar). The total amount of radiocesium (Bq/tissue) was approximately equal between the outer (bark) and the inner (sapwood and heartwood) parts of the tree.
To understand how 134Cs and 137Cs move within a fruit tree, a contaminated peach tree was transplanted to a noncontaminated site after its twigs, leaves, and fine roots were removed. One year later, all of the newly developed tissue (including the fruit) was harvested, and the radioactivity was measured. As shown in Table 1, only 2% of 134Cs and 137Cs that moved inside the bark had transferred to the newly developing tissue, including the roots. Therefore, 97% of 134Cs and 137Cs that had accumulated inside the tree did not move. Approximately 0.6% of 134Cs and 137Cs inside the tree moved to the fruit.37)–39)
| 137Cs (Bq/tissue) | % of movement* | |
|---|---|---|
| New roots | 27.7 ± 2.2 | 0.46 |
| Meristems | 15.0 ± 1.9 | 0.25 |
| New leaves | 47.1 ± 1.5 | 0.79 |
| Fruits | 37.1 ± 2.5 | 0.62 |
*137Cs accumulation in tree was 5980 Bq (2011).
Although milk supplied to consumers was free from 134Cs and 137Cs in the first year after FNPA, experiments were conducted to determine if milk could become contaminated by feeding contaminated feed to dairy cows. Therefore, contaminated haylage was prepared and supplied to dairy cows, and the radioactivity of their milk was measured. The milk was found to contain 134Cs and 137Cs soon after the contaminated feed was supplied. The radioactivity content of the milk reached a plateau after approximately 14 days. The contaminated feed was then replaced with noncontaminated feed. As soon as the noncontaminated feed was supplied, the radioactivity in the milk decreased, and the value returned close to the background level after 14 days (Fig. 11A).40) These results on milk contamination have subsequently been adopted as official data for the Ministry of Agriculture, Forestry and Fisheries in Japan. Similar results were found for animal meat, indicating that when contaminated animals were identified, radioactivity in the living animal could be rapidly decreased by feeding with noncontaminated feed. The biological half-life of 137Cs was estimated to be less than 100 days because of animal metabolism, whereas its physical half-life is 30 years. However, a newborn calf delivered from a contaminated cow had a similar level of radioactivity (Fig. 11B).
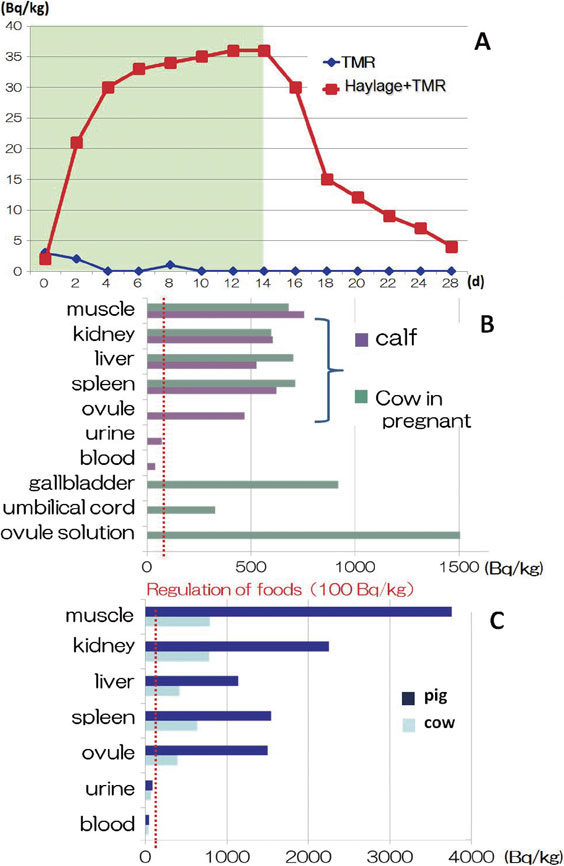
Radioactivity in milk and animals. A: 137Cs concentration in milk. Contaminated haylage was supplied to the cows for two weeks, and then noncontaminated feed was supplied for a further two weeks. The radioactivity of the cow’s milk was measured throughout the four-week trial. B: Radioactivity of tissues, urine, and blood of a pregnant cow and her newborn calf. C: Radioactivity of tissues, urine, and blood of a pig and cow.40)
When the contamination level of cows was compared to that of pigs, the level in pigs was distinctly higher. It was suspected that pigs consume a considerable amount of contaminated surface soil while foraging, while cows eat only freshly grown grass. This difference in eating behavior may impact the extent of the contamination in these two farm animals (Fig. 11C).40),41) Additionally, in the highly contaminated areas of Fukushima prefecture, natural matings between pigs and wild boars are taking place, and the number of hybrid animals is increasing. Higher 134Cs and 137Cs levels are observed in these pig-boar hybrids and wild boars than in cows (K. Tanoi, personal communication).
The most effective and efficient way to prevent 134Cs and 137Cs uptake in crops is to apply K fertilizer on farmland. Other chemicals such as Prussian blue or zeolite, which are known cesium adsorbents, are too expensive for agricultural use. Because agricultural soil is a very important natural resource, the soil surface removal cannot be recovered simply by introducing other soils. The best decontamination method is to eliminate only the contaminated particles in the soil. 134Cs and 137Cs were only found to be adsorbed firmly on the fine clay component of soil. Therefore, one decontamination trial was conducted as follows. First, water was introduced into a contaminated field and mixed well with the surface soil. Once the soil component had precipitated, the suspended fine clay particles in the water were drained off into an adjacent ditch in the field. Most of the soil component was deposited in the original field, with only the radioactive clay suspended in the supernatant being removed. Thus, more than 80% of the radioactivity in the field was removed (Fig. 12).42) Although the decontamination rate depended on the condition of the farmland, this method was adopted as a recommendation by the Ministry of Agriculture, Forestry and Fisheries in Japan.

Radiocesium decontamination of an agricultural field. Because radiocesium is mostly adsorbed to fine clay (A), water was introduced into the field and mixed well (B). The supernatant was allowed to drain into a ditch adjacent to the edge of the field. Approximately 80% of the radioactivity in the field was removed (C), and most of the soil remained in the field.42)
The behavior of 134Cs and 137Cs emitted from the nuclear accident was different from that of known macroscopic cesium chemistry. Because the amount of radiocesium deposited on the leaves was so small and carrier-free, the nuclides behaved like radiocolloids or as if they were electronically adsorbed onto the tissue.
The important findings were as follows.
The downward movement was only 1–2 mm/year.
Many scientific findings have been accumulated from this work, and the results above summarize only a portion of the total research.
Because Japan is located in a monsoon area with many paddy fields for rice, its agricultural environment is similar to those of other Asian countries but different from that of Chernobyl. Therefore, it is important to compile data regarding the movement of radioactive material and features of fallout found in Japan and disseminate the findings to relevant stakeholders around the world, especially those concerned with agriculture.
Research continues in the hope that these results will be useful to the recovery of the affected area in Fukushima.

Tomoko M. Nakanishi was born in 1950 in Kanazawa and received her Ph.D. in 1978 for determining the half-lives of two long-lived nuclides, 92Nb and 91Nb, under the supervision of the late Professor Masatake Honda in the Department of Chemistry at the University of Tokyo. She started her career as an assistant professor in the Department of Agriculture at the University of Tokyo in 1987 and became a full professor in 2001. She is known for her original work using radioactive nuclides and a neutron beam for imaging. Using 15O-labelled water, she showed for the first time that water absorbed from roots circulates in the plant stem and is renewed by freshly absorbed water within 20 min. She has also been developing real-time macroscopic and microscopic imaging systems using commercially available nuclides, as well as producing 28Mg and 42K in her own laboratory, which she has used for the first time as tracers. After the Fukushima nuclear plant accident, she became a leader among the faculty in studying the agricultural impacts of radioactive contamination. She has edited two books on this topic, published by Springer in 2013 and 2016, which to date have been downloaded 110,000 and 40,000 times, respectively. She has received the Saruhashi Award (given to the premier female scientist in Japan), the Japan Soc. of Nuclear and Radiochemical Sciences Award, L’ordre national du Mérite (from the French president), the Hevesy Medal Award, etc. She was a member of the Science Council of Japan and is currently the president of the Japan Soc. of Nuclear and Radiochemical Sciences, vice president of the Engineering Academy of Japan, a foreign member of the Royal Swedish Academy of Engineering Sciences, and a commissioner of the Japan Atomic Energy Commission.
This report wraps up the official project activities performed at the Graduate School. The author is very thankful to all of the faculty and graduate students who performed research related to the FNPA.