2019 Volume 95 Issue 3 Pages 136-149
2019 Volume 95 Issue 3 Pages 136-149
Since globotetraosylceramide was defined as a major glycosphingolipid in human erythrocytes, various glycolipids have been found in normal cells and diseased organs. However, the implications of their polymorphic structures in the function of individual cells and tissues have not been clarified. Genetic manipulation of glycosphingolipids in cultured cells and experimental animals has enabled us to substantially elucidate their roles. In fact, great progress has been achieved in the last 70 years in revealing that glycolipids are essential in the maintenance of integrity of nervous tissues and other organs. Furthermore, the correct composition of glycosphingolipids has been shown to be critical for the protection against inflammation and degeneration. Here, we summarized historic information and current knowledge about glycosphingolipids, with a focus on their involvement in inflammation and degeneration. This topic is significant for understanding the biological responses to various stresses, because glycosphingolipids play roles in the interaction with various intrinsic and extrinsic factors. These findings are also important for the application of therapeutic interventions of various diseases.
Communicated by Kunihiko SUZUKI, M.J.A.
Dedicated to the late Dr. Tamio Yamakawa.
Abbreviations: DKO: double knockout; DRM: detergent-resistant microdomain; ES: embryonic stem; FA: fatty acid; Gb3: globotriaosyl ceramide; Gb4: globotetraosylceramide; GEM: glycolipid-enriched microdomain; GPI: Glycolipid phosphatidylinositol; HSP: hereditary spastic paraplegia; HUS: hemolytic uremic syndrome; IL: interleukin; iPS: induced pluripotent stem; KO: knockout; LPS: lipopolysaccharide; TNFα: tumor necrosis factor alpha.
A number of studies on the expression and structures of glycosphingolipids have been performed to date,1) suggesting that polymorphic forms of glycosphingolipids should play roles in the regulation of cells and tissues, and eventually our bodies.2) Efforts to clarify the functions of glycosphingolipids have spanned many years. Numerous studies, including our own, have revealed that glycosphingolipids are essential in the maintenance of integrity of nervous tissues and other organs and in the regeneration of injured nerves.3)–5) Furthermore, the correct composition of glycosphingolipids has been shown to be critical for protection against inflammation not only in nervous tissues but also in other tissues such as the vascular system.6) In this review, we summarize the accumulated heritage of previous studies and current knowledge about glycosphingolipids with a focus on their involvement in inflammation and degeneration, as demonstrated by the manipulation of glycosyltransferase genes in cultured cells and experimental animals. There seems to be great significance in understanding the biological responses to various stresses, particularly because glycosphingolipids are mainly present in the membrane of almost all cells, and they play roles in interactions with various intrinsic and extrinsic factors and other cells. These findings are also important in considering their application in therapeutics for various tissue and cell disorders.
In 1951, Yamakawa’s group detected globotetraosylceramide (Gb4) as a major component among glycosphingolipids in human erythrocytes.7) The chemical structure of the molecule was soon clarified in 1952–1953 by the same group,8) and the component was designated “globoside” based on the supposed molecular shape. Namely, its round morphology (globular form) became its chemical nomenclature.9) Thus, globoside was the first glycosphingolipid defined as a human blood group glycolipid antigen.10),11) After discovery of globoside, no definite function was reported for more than 65 years, even though the amount of globoside in human erythrocytes is unusually high among the glycolipids in various human cells and tissues. Since then, various structures of glycosphingolipids present in the body have been found, and the synthetic pathways of them including globo-series have also been defined (Fig. 1). The enzymes and genes responsible for the synthesis of precursors for these structures have all been defined.12),13)

Gb3 synthase (A4GALT in human or A4galt in mouse), which is responsible for the synthesis of globotriaosyl ceramide (Gb3) and eventually of all globo-series glycolipids, was isolated14) and the genetic basis for blood group pk/p was clarified.15),16) Surprisingly, the basis for P1/P2 phenotypes was also attributed to the A4GALT gene, for which a regulatory sequence of the gene was involved together.17) Furthermore, definitive evidence for the biological roles of globo-series glycosphingolipids has been demonstrated using the genetic manipulation of glycosyltransferase genes in cultured cells and experimental animals (Fig. 2).6),18),19) Gb4 synthase cDNA20) (B3GALNT1 in human or B3galnt1 in mouse), Gb5 synthase cDNA21) (B3GALT5 in human or B3galt5 in mouse), and sialyl Gb5 synthase cDNA22) (ST3GAL2 in human or St3gal2 in mouse) were also cloned subsequently. The expression and functions of the individual structures shown in Fig. 2 are described in details in the following sections.

Globo-series glycosphingolipids. Globo-series glycosphingolipids are generated via Gb3 with the A4galt enzyme.14) Extended forms of globo-series glycolipids are expressed in restricted cells and organs such as erythrocytes (human),7),15)–18) kidney,19) endothelial cells,6),19) B lymphocytes (some differentiation stages),25)–28),34) early developmental stages of embryonal cells,28),30),31) and some malignant tumor cells.23),24) Cytosolically located GlcCer is converted to lactosyl ceramide (LacCer) by B4galt5/B4galt6 in the Golgi,13) and then Gb3 is synthesized in the Golgi by A4galt. It is transferred and expressed in the outer leaflet of membranes.
It was shown that Gb3 is specifically expressed in Burkitt’s lymphoma cells.23) Wiels et al. demonstrated that Gb3 is a Burkitt-associated glycolipid antigen by raising a monoclonal antibody.24) It was also reported that Gb3 was expressed in a restricted population of B lymphocytes at particular differentiation stages, such as in a proportion of tonsil lymphocytes.25) Furthermore, it was reported that Gb3-positive B cells were assigned to undergo apoptosis, thereby specifying Gb3 as a death-marker.26) Although the actual roles of Gb3 in the determination of B cell fates have not been clarified, Gb3 is expressed in the activated population27) or some differential stages of B lymphocytes.28)
Furthermore, Gb3 was also detected in human megakaryocytes.18) Gb3/Gb3 synthase was induced along with megakaryocyte differentiation, and high levels of Gb3 were detected on the surfaces of platelets. It seems unexpected that the function of Gb3 expression on platelets has not yet been reported. Additionally, whether platelets are damaged by verotoxins has not been reported to date.
2-3. Globo-series glycolipids in non-hematopoietic cells.Of interest is that some globo-series glycolipids are expressed at an early stage of development, and these are being used as markers for stem cells, e.g., SSEA3 and SSEA4.29) In particular, SSEA4 has been used as a “Venus cell” marker to isolate potential stem cells.30),31) From the phenotypes of a gene knockout (KO) of A4galt, the significance of these markers seems not to be critical for the nature of the stem cells and developmental processes. However, they may play important roles in the fine regulation of differentiation and/or maintenance of “stemness”.
As described above, globo-series glycolipids are used as markers for stem cells. Embryonic stem (ES) cells and/or induced pluripotent stem (iPS) cells are expected to be utilized in regenerative medicine by being induced to form various differentiated and functional cell lineages.32) In the clinical application of these “stem cells” to reconstruct lesioned sites in the body, the presence of undifferentiated ES/iPS cells in the administered cells is the most critical issue, because they may cause neoplasms.33) Therefore, useful markers to distinguish undifferentiated stem cells have been sought, among which carbohydrate markers have been widely used. For instance, SSEA3 and SSEA4 are glycolipids belonging to the globo-series. Other glycosylation markers of stem cells have also been defined, and these have been verified as useful for eliminating contaminating stem cells.34)
Globo-series glycolipids are also present on endothelial cells. It has long been known that inflammatory stresses such as tumor necrosis factor alpha (TNFα), interleukin (IL)-1β, and IL-6 could induce the expression of globo-series glycolipids on endothelial cells as demonstrated using human umbilical vein endothelial cells (HUVECs).35)–37) If endothelial cells are exposed to these inflammatory cytokines during infection by E. coli. O157, the induced globo-series glycolipids may enhance cytotoxicity towards the vascular system.
The definitive biological function of Gb3 is as a receptor for verotoxins.38) Since Lingwood et al. reported that Gb3 is a receptor for Shiga-like toxins, which are verotoxins from pathogenic E. coli, O-157, this has been believed by many researchers despite a lack of definite evidence. Verotoxins are A1B5 type bacterial toxins similar to cholera toxin.39) These toxins commonly consist of one A subunit and five B subunits.40) The A subunit has toxic effects, whereas the B subunit attaches cellular receptors to induce subsequent invasion of the A subunit into cells.
We established KO mice deficient in Gb3 synthase (A4galt1) and examined their sensitivity to verotoxins.19) Consequently, it was demonstrated that globo-series glycolipids are specific receptors for verotoxins. A4galt1-deficient mice were completely resistant to injected verotoxins even at 1000 times more than the lethal dose for wild-type (WT) mice.19) In particular, we investigated the mechanisms for disturbed consciousness in hemolytic uremic syndrome (HUS), which is caused by severe O157 infection. Patients with HUS often exhibit unconsciousness, although no globo-series glycolipids have been found in brain tissues. Verotoxin-injected mice showed brain edema, resulting in brain damage. The immunohistostaining of brain tissues with an anti-Gb3 antibody as well as anti-CD31 revealed that Gb3 was expressed together with CD31 only in endothelium in the brain tissues, but not in neural cells. These results suggested that brain disorders in HUS might be due to damage to the vascular system, in which globo-series glycolipids are expressed abundantly.41)
In addition to the receptor function for verotoxins, globo-series glycolipids have been reported to play a role as a receptor for pig edema toxin.42) Thus, many glycosphingolipids serve to receive invading pathogens and pathogen-derived toxins,43)–45) inducing infectious diseases and/or serious toxin diseases.43) The roles of individual glycosphingolipids in infection and inflammation have been further examined using various glycosyltransferase gene KO mice.46)–48)
3-2. Gb4 as an endogenous ligand molecule for LPS receptor, TLR4/MD2.As described above, inflammatory stresses such as TNFα, IL-1β, and IL-6 induced the expression of globo-series glycolipids on endothelial cells such as HUVECs.35)–37) This fact prompted us to examine the sensitivity of A4galt1-KO mice to lipopolysaccharide (LPS). Unexpectedly, globo-series-glycolipid-deficient mice were more sensitive to injected LPS than WT mice,6) indicating some or all globo-series glycolipids play roles in suppressing the signals induced via the LPS receptor, TLR4/MD2.6) Based on the expression analysis of glycolipids and glycosyltransferase genes responsible for the synthesis of globo-series glycolipids, globoside was defined as a molecule that bound to TLR4/MD2 among those expressed in LPS-stimulated endothelial cells.6) The binding of globoside with TLR4/MD2 was specific as verified by a co-precipitation assay and a native gel electrophoresis assay.6) Here, we defined that TLR4/MD2 is the first example of an endogenous ligand of globoside. More than 60 years after its discovery by Yamakawa et al.,7) its possible implications in protection against bacterial invasive damage have been demonstrated. Thus, globoside has encountered genuine endogenous ligands in this era of globalization, where globoside is known as the biggest component in any cells or tissues in our bodies (Fig. 3).

Globo-series glycolipids interact with exogenous and endogenous factors on the cell surface. Gb3 specifically binds the B subunit of verotoxins, playing a role as a receptor for exogenous bacterial toxins,19),38),41) whereas Gb4 binds TLR4/MD2 as an endogenous ligand. Gb4 with saturated FAs binds the gap region in MD2 molecules, competing with LPS in the binding of its receptor.6)
Surprisingly, mass spectrometry of MD2-binding glycolipids in an endothelial cell line revealed that only restricted molecular forms of globoside could bind MD2, i.e., globoside with linear (saturated) fatty acids (FAs) such as C24:0 and C16:0 were co-precipitated by MD2-tagged proteins, but unsaturated FA-containing globoside was not. Molecular modeling also verified that only globoside with linear FAs could be accommodated in the gap region of the MD2 molecule.6) Furthermore, in silico docking of Gb4 to MD2 performed using Molecular Operating Environment (MOE) revealed that the lipid IVa binding site on MD2 may be shared with Gb4, and that the ceramide moiety of Gb4 was embedded in the hydrophobic cavity of MD2 as lipid IVa.6) Consequently, not only the ceramide portion of Gb4 but also its carbohydrate moiety showed significant hydrogen bonds to be reactive with several amino acids in TLR4-MD2 and MD2.6)
In a trial to clarify the possibility of clinical applications of globoside in the treatment of endotoxin shock, administration of globoside into semi-lethal dose-glucosamine-injected mice could prevent lethality and alleviate liver toxicity.6) Taken together with results of binding experiments, the potential application of particular molecular species of globoside was suggested to be promising for the treatment of endotoxin shock.6)
The functions of globo-series glycolipids elucidated so far are summarized in Fig. 4.
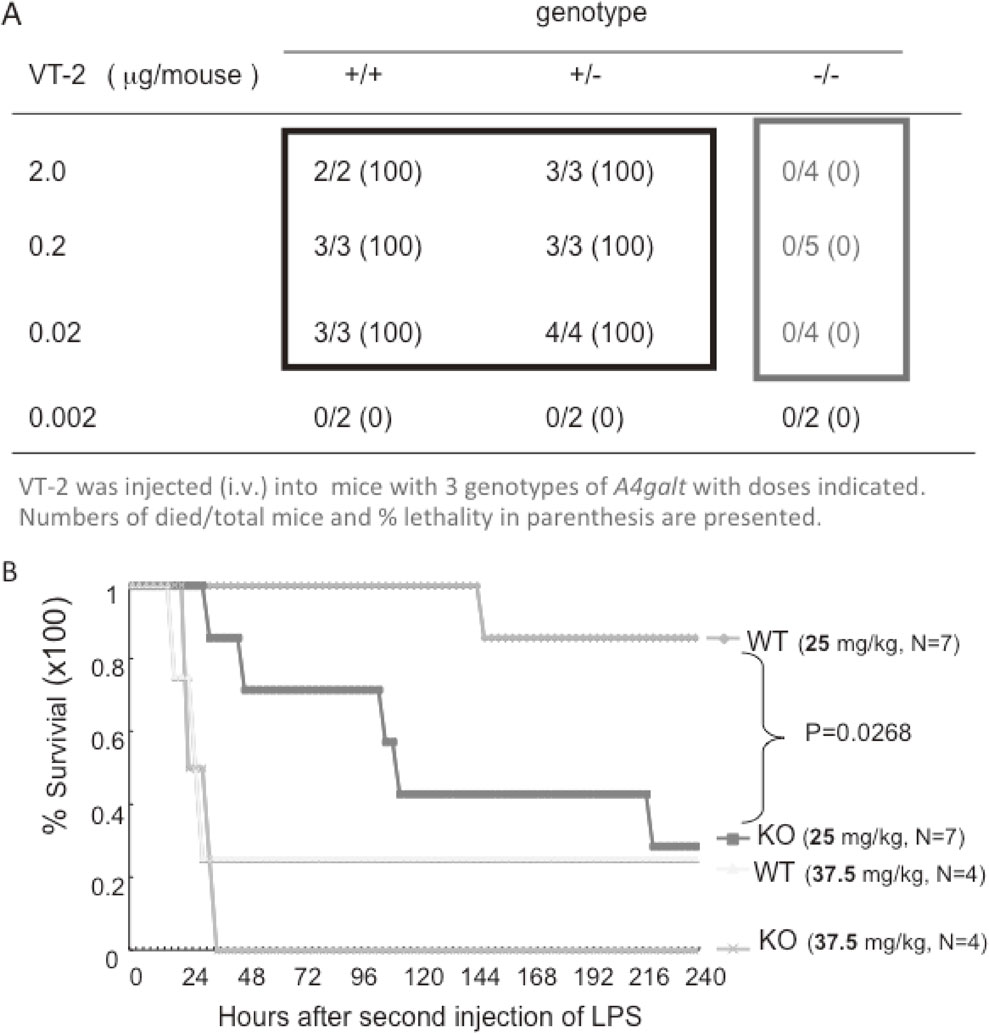
Function of globo-series glycosphingolipids elucidated in Gb3 synthase (A4galt)-KO mice. A, Gb3 synthase KO mice were resistant to verotoxins. These KO mice were resistant to VT-2 at more than a 100 times higher dose than is lethal for WT mice.19) B, Gb3 synthase KO mice were more sensitive to LPS (Shwartzman, R), as shown by the percentage survival after the injection of LPS. Particularly when LPS was injected at 25 mg/kg mouse, a significantly lower survival rate was observed in Gb3 synthase KO mice than in WT mice.6)
A simple ganglioside GM3 is widely distributed in almost all tissues and cells in vertebrates.49) NeuGc-type GM3 is found abundantly in horse erythrocytes, as determined by Yamakawa et al.50),51) In turn, NeuAc-type GM3 is found abundantly in erythrocytes in the majority of dogs. Human erythrocytes also contain fairly high levels of NeuAc-type GM3. The majority of gangliosides are synthesized via GM3, and diverse carbohydrate structures are formed along with several major core structures.52) A lack of GM3 synthesis in humans results in serious infantile disorders, mainly in nervous tissues, muscles,53) and skin,54),55) suggesting that gangliosides are generally essential in the maintenance of correct body function. Nevertheless, GM3 synthase (St3gal5)-disrupted mice showed almost normal appearance and growth after birth. The reasons for differences in the effects of loss of GM3 synthase activity between human and mice have not yet been clarified.
4-2. Disruption of ganglioside synthesis results in aging-dependent inflammation and degenerative disorders.In order to understand the biological functions of individual glycolipid structures, we established various glycosyltransferase-disrupted mutant mice.5),56) In GM2/GD2 synthase (B4galnt1) KO mice, no clear defects in nervous tissues and other organs were detected except aspermatogenesis in the early stages of life.57),58) However, GM2/GD2 synthase KO mice showed increasing neurological disorders with aging.59) Marked astrocytosis with giant processes surrounding blood vessels and neuron degeneration with modified morphology of synapses and spines were observed.56) Accordingly, KO mice showed reduced sensory functions and subsequent motor neuron deficits. A mild increase in the expression levels of complement genes was observed. In turn, myelin-associated glycoprotein-ganglioside binding ensures long-term axon-myelin stability, because B4galnt1-null mice displayed axon degeneration in the central nervous system and peripheral nervous system, and reduced axon caliber, reduced axon neurofilament spacing, and motor behavioral deficits.60) However, the abnormal phenotypes that GM2/GD2 synthase KO exhibited were much milder than expected despite the disappearance of whole complex gangliosides.57) Remaining GM3 and GD3 showed tremendous increase in their amounts, and these might compensate for the defect resulting from the lack of complex gangliosides.
In order to clarify the significance of gangliosides in vivo, we established double KO (DKO) mice of GM2/GD2 synthase and GD3 synthase (St8sia1) genes in order to eliminate the effects of the remaining structures as much as possible. Namely, only GM3 among gangliosides remained in these DKO mice. Thus, we designated them as “GM3-only mice”.61) They showed various serious phenotypes from early life stage after birth. They showed disturbed sensory and motor neuron functions in early growth stages and started to die gradually after 12 weeks after birth. As shown in Fig. 5A, it was characteristic that DKO mice exhibited refractory skin lesions on the face and tips of the fingers probably due to reduced peripheral pain sensation and frequent scratching of themselves. All these disorders seemed to be derived from systemic nerve degeneration.

DKO mice exhibited refractory skin lesions on the face and tips of the fingers. A, TLC pattern of gangliosides from the brains of WT and DKO mice. Degeneration of sciatic nerve in DKO (lower) compared with WT (upper) mice. Skin lesions on the face and the tips of the fingers in DKO mice.61) Arrows indicate injured and bleeding fingers. B, Progressive inflammatory reactions with aging as detected using the mRNA expression levels of inflammatory cytokines (IL-1β) and complement. RNA was extracted from the cerebella of DKO mice (GM2/GD2 synthase and GD3 synthase) as shown by - ◆ -, and by - ● - for WT.62),67),68) C, A scheme to summarize the effects of ganglioside deficiency on inflammation and neurodegeneration, and rescue of these pathological processes by the loss of complements (TKO).
To clarify the mechanisms of neurodegeneration in ganglioside-deficient mice, gene expression profiling was performed using a DNA microarray.62) Among 25 genes that showed 4-fold increase in their expression levels in DKO mice compared with in WT mice, about half were inflammation-related genes, such as S100A, and several complement components and their receptors. These results suggested that ganglioside deficiency induced neuroinflammation in brain tissues, as shown in Fig. 5B. We focused on cerebella and spinal cords and investigated complements, inflammatory cytokines, and inflammatory glial proliferation. Consequently, we determined that ganglioside deficiency induces inflammatory reactions in brain tissues, such as gliosis, microglia assembly, increased inflammatory cytokine production, and increased consumption of complement components. These inflammatory reactions became more severe with aging, and corresponded well with progressive neurological disorders such as gait disturbance, tremor, and memory loss with progressive exacerbation with aging (Fig. 5C).63)
4-4. Microdomain architecture and functions regulate membrane integrity.Membrane microdomains such as glycolipi-enriched microdomains (GEMs)/rafts or detergent-resistant microdomains (DRMs) have been considered to be a platform to regulate endocytosis, exocytosis, infection, and various growth/adhesion signals.64),65) Glycosphingolipids as well as cholesterol and sphingomyelin are the main resident molecules in lipid rafts, as shown in Fig. 6A.66) Glycolipid phosphatidylinositol (GPI)-anchored proteins are also localized in lipid rafts, where they have their roles. The effects of ganglioside remodeling on GEM/rafts were analyzed using several KO mouse lines of ganglioside synthase genes. One important points to be considered here is that many of complement-regulatory molecules are GPI-anchored proteins, suggesting that their localization in GEM/rafts might be critical for their function. Consequently, the architecture and functions of GEM/rafts were largely affected by the range of deleted ganglioside structures in individual mutant mice.62) As shown in Fig. 6B, the localization of resident molecules such as DAF and CD59 containing GPI-anchors as well as caveolin-1 and flotillin-1 were dramatically shifted from GEM/rafts to non-GEM/rafts fractions in the brain tissues of DKO mice, and moderately in single gene KO mice.67) Thus, it was assumed that dislocation of GPI-anchored proteins caused reduced regulatory function of complement system, leading to the complement activation and subsequent inflammatory reaction in the mutant mice.

Glycosphingolipids play roles in the maintenance of the integrity of nervous tissues. A, Lipid/rafts (GEM/rafts) consist of sphingomyelin, glycosphingolipids, and GPI-anchored proteins.65),66) Various growth factor receptors and adhesion receptors are frequently localized in lipid/rafts depending on the cellular situation. B, Shifts in raft-resident molecules to non-raft fractions due to ganglioside deficiency. Neurodegeneration is induced by disordered GEM/rafts in ganglioside-deficient mice.62),67)–69)
To confirm this hypothesis, a triple knockout (TKO) mouse line was established by mating C3-deficient mice and DKO mice (GM3-only mice). In TKO mice, the majority of noticeable features found in DKO mice were largely mitigated, i.e., proliferation of astrocytes, assembly of microglia, increased complement factors, increased inflammatory cytokines, and morphological changes, such as deletion of Purkinje cells in cerebellum.62),67) These results suggested that gangliosides are essential to maintain the integrity of the nervous systems based on the regulation of GEM/rafts, and disturbed ganglioside composition induced inflammatory reaction and subsequent neurodegeneration due to disordered GEM/rafts,68),69) as summarized in Fig. 5C and Fig. 6.
All these results described above suggested that sound membrane functions in nervous tissues are regulated and protected by a highly controlled composition of glycosphingolipids. Namely, we would propose here “self-defense of nervous tissues” with complex gangliosides, which continuously regulate the cell membrane and GEM/rafts by flexibly responding to intrinsic and extrinsic changes in microenvironments. Thus, the integrity of GEM/rafts is essential for the protection of inflammation and neurodegeneration.70)
As described above, the majority of gangliosides are synthesized through GM3, and diverse carbohydrate structures are generated from a common precursor, lactosylceramide, along with several major synthetic pathways.52) Congenital defects in GM3 synthase gene in humans were found in Amish families.53) They exhibited serious infantile epileptic disorders53) and skin abnormalities,54),55) suggesting that gangliosides are essential in the regulation of nervous tissues and other organs. On the other hand, GM3 synthase (St3gal5 in mouse)-KO mice showed no apparent disorders in any sites in the body, including nerve tissues, except for the auditory systems.71)
5-2. Common features of neuronal disorders in patients with hereditary spastic paraplegia due to mutated B4GALNT1 gene and in KO mice of B4galnt1.B4GALNT1 is an essential enzyme for the synthesis of complex gangliosides, lack of which resulted in progressive neurodegeneration with aging in mice. Recently, 11 cases of HSP due to mutation in the coding region of B4GALNT1 were reported.72)–74) We examined the enzyme activities using a cell free assay, and by flow cytometry of transfectant cells with mutant cDNA expression vectors. Among them, almost all mutant genes showed complete loss of B4GALNT1 activity, whereas two mutants showed low activity. Considering the clinical findings from these patients, loss of enzyme activity should be responsible for the clinical signs of HSP, whereas the intensity of their neurological disorders were milder than expected. These clinical features of the patients including male hypogonadism are very similar with abnormal phenotypes detected in B4galnt1-deficient mice75) as shown in Fig. 7. Many of these abnormal features might result from disordered neuron-glia communication,76) whereas lack of glycosphingolipids brought about much more severe phenotypes even with neuron-specific conditional KO.77),78) In contrast to GM3 synthase deficiency, B4galnt1-deficient mice could be suitable models for HSP.

Hereditary spastic paraplegia (HSP) and abnormal phenotypes in B4galnt1-deficient mice. Patients with HSP with a mutated B4GANT1 gene exhibited relatively mild neurological disorders.72)–74) These clinical features were similar to the phenotypes of B4galnt1 KO mice.57),58) Including male infertility, B4galnt1 KO mice might be good models for HSP.75)
As mentioned in the beginning, the ganglioside compositions in nervous tissues are well preserved among species,79) suggesting their universal roles. During development, the expression profiles of gangliosides in brain tissues change dramatically, whereas they show a stable composition after birth. However, there are minor changes in ganglioside composition even in adulthood. The meaning of dynamic changes in ganglioside profiles in a spatio-temporal manner is not yet well understood.80) However, all these changes should contain individually reasonable purposes for the maintenance of integrity and homeostasis in the bodies based on fine tuning by glycosylation.81)
5-4. Modulation of glycosphingolipids as a therapeutic strategy.Changes in the composition of gangliosides could be causes of inflammatory reaction and neurodegeneration; therefore, the artificial modification of ganglioside synthesis and expression may be potential therapeutic approaches.82) Although it is not a main theme in this review, a number of altered glycosphingolipids in malignant cancers have been reported, and a substantial basis for the metabolic changes in glycosylation in cancer cells have been elucidated.70) Thus, modulation of glycosphingolipids based on the modification of their synthetic machinery should be a main target in glycoscience research, not only for the regulation of inflammation and degeneration but also of the control of malignant tumors.
Since Gb4 was discovered on human erythrocytes, a number of glycosphingolipids have been identified, and their expression patterns have been widely analyzed. In the last few decades, however, dramatic progress in the understanding of glycopshingolipids has been achieved based on molecular biology approaches. Novel technologies for high-resolution imaging, chemical synthesis, structure analysis, and structure biology, etc. have also promoted major progress in analysis of the functions and modes of action of glycosphingolipids. The mysteries of glycosphingolipids are now being elucidated day by day.

Koichi Furukawa was born in 1949 in Gifu. He graduated from Nagoya University School of Medicine in 1975. After clinical training, he majored in hematology at the Department of Internal Medicine, Nagoya University. He received his M.D./Ph.D. degree in 1984. He studied at Memorial Sloan-Kettering Cancer Center, New York, as a Research Associate between 1984 and 1989 under the supervision of Dr. Kenneth O. Lloyd and Dr. Lloyd J. Old. He worked on the identification of cancer-associated glycolipids. Then, he worked as an Assistant Professor (and later an Associate Professor) at the Department of Oncology, Nagasaki University School of Medicine, with Prof. Hiroshi Shiku between 1989 and 1997. Here, he cloned cDNAs for many glycosyltransferase genes, opening a new dimension of glycoscience. He was appointed as a Professor in the Department of Biochemistry II, Nagoya University School of Medicine (later Graduate School of Medicine) in 1997, and worked there until 2015. His main studies were on the functions of complex carbohydrates, particularly of glycosphingolipids in cancer and the brain. He demonstrated the essential roles of cancer-associated glycolipids in the malignant properties of cancers and also the pivotal roles of glycosphingolipids in inflammation and neurodegeneration based on the regulation of lipid/rafts. He served as a representative of a Grant-in-Aid for Scientific Research on Priority Areas of MEXT, “Functional Glycomics” between 2002–2007. From 2015, he has worked in Chubu University College of Life and Health Sciences to extend glycoscience as President of the Research Institute of Life and Health Sciences in Chubu University.