2019 Volume 95 Issue 9 Pages 538-567
2019 Volume 95 Issue 9 Pages 538-567
Translation is the process of turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and the public, ranging from diagnostics and therapeutics to medical procedures and behavioral changes. Translational research is defined as the effort to traverse a particular step of the translation process for a particular target or disease. Translational science is a newly emerging science, distinct from basic and clinical sciences in biology and medicine, and is a field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process. Advances in translational science will increase the efficacy and safety of translational research in all diagnostic and therapeutic areas. This report examines translational research on novel hormones, the natriuretic peptide family and leptin, which have achieved clinical applications or for which studies are still ongoing, and also emphasizes the lessons that translational science has learned from more than 30 years’ experience in translational research.
Edited by Hiroo IMURA, M.J.A.
Linguistically, the term “translation” refers to the conversion of one language to another language. The term is also used to refer to the synthesis of proteins from mRNA in biology. Since the last decade of the 20th century, the terms “translational research” and “translational science” have been used to refer to the process or concept by which we move from discoveries to applications in medicine.1)–3) Because progress in biology was rapidly applied to medicine in the 20th century, the term “translation” has become widely used in biology and medicine under the proposal of the National Center for Advancing Translational Science (NCATS), resulting in divergence from the original conceptual meaning.
The original definition of translation refers to the process of turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and the public, ranging from diagnostics and therapeutics to medical procedures and behavioral changes. Translational research is defined as the effort to traverse a particular step of the translation process for a particular target or disease. Translational science is a newly emerging science, distinct from basic and clinical sciences in biology and medicine and is a field of investigation focused on understanding the scientific and operational principles underlying each step of the translational process.
Despite rapid and remarkable advances in medical science, especially in basic science, discoveries in many steps of the translation process are only being introduced to clinical applications after a time-consuming process, and only in extremely rare cases. The process has often been likened to a seemingly endless trip through a long, dark tunnel. These challenges withstanding, the research field of translational science is anticipated to transform discoveries into clinical applications in medicine and make innovative medical technologies a reality. Above all, advances in translational science will increase the efficacy and safety of translational research in all diagnostic, therapeutic, and behavioral applications.
Although a multitude of animal models have been developed to emulate various human diseases, there are only a few animal models that mimic human disease remarkably accurately, e.g., spontaneously hypertensive rats (SHRs)4) and hereditary obese mice, (ob/ob)5) are very useful for the preclinical steps of translational research on hypertension and obesity, respectively. Lessons from research history on SHRs, an excellent animal model for hypertension research, led us to investigate the clinical significance of the natriuretic peptide family [atrial natriuretic peptide (ANP), brain natriuretic peptide (BNP), and C-type natriuretic peptide (CNP)] in SHR in the early stages of preclinical research. For translational research on leptin, a representative adipokine, in addition to obese mouse models such as the leptin knockout (KO) ob/ob mice and leptin receptor KO db/db mice, we generated leptin transgenic (Tg) “skinny” mice with elevated plasma leptin concentrations similar to those in obese humans and assessed the functional and therapeutic implications of leptin beyond species differences. These studies led to establishment of a “proof-of-concept (POC)” that plays a pivotal role in the transitional step from the preclinical stage to clinical trials of the translational science spectrum.
This review summarizes the current state of translational research on the natriuretic peptide family (ANP, BNP, and CNP), which are prototype cardiovascular hormones (Fig. 1), and on leptin, a representative adipokine (Fig. 2 and Table 1). In addition, general principles of translational science are proposed that have accumulated in the course of translational research. Suitable animal disease models mimicking human diseases are indispensable in the preclinical stage. Target diseases for the initial step of clinical trials could be rare human diseases, and then the target diseases expand from rare human diseases into common human diseases that are essentially close to rare human diseases in their etiology and pathophysiology.2)

Cardiovascular hormones and the cardiovascular system as an endocrine organ. Members of the natriuretic peptide family are prototype cardiovascular hormones.

Adipocyte-derived factors, adipokines, released from adipocytes. Adipose tissue is a novel endocrine organ. Leptin is an adipokine that induces severe obesity when depleted.
| Application | State/Industry | Disease and Target | |
|---|---|---|---|
| ANP | Therapeutic | Achieved/Suntory (Daiichi-Sankyo) | Congestive heart failure |
| Diagnostic | Achieved/Shionogi | Body fluid volume | |
| BNP | Diagnostic | Achieved/Shionogi | Heart failure and hypertrophy |
| CNP | Therapeutic | Ongoing/Chugai Pharmaceutical | Achondroplasia |
| Leptin | Therapeutic | Achieved/Shionogi | Lipodystrophy |
| Diagnostic | Achieved*/Cosmic Corporation | Lipodystrophy, Adiposity |
*Diagnostic application of leptin for lipodystrophy has been approved by PMDA after the submission of the manuscript.
Progress in biology was rapidly applied to medicine in the 20th century, and the fields explored by basic scientists and physicians or clinicians became much wider, deeper, and more separate. It is very hard for medical doctors to be experts in both basic or preclinical research and clinical research or medicine (Fig. 3). From the last decade of the 20th century, the terms “translational research” and “translational science” have been used to describe the concept of the process of moving from a discovery to clinical practice.1)–3) The term “translation” has been widely used in biology and medicine (i.e., biomedicine), under a proposal of the NCATS, resulting in divergence in the conceptual meaning relative to the previous 30 years.

Effects of progress in medicine on basic scientists, clinician-scientists (translational scientists), and clinicians or clinical scientists.
According to the original definition by NCATS,3),6) translation is the process of turning observations in the laboratory, clinic, and community into interventions that improve the health of individuals and the public, ranging from diagnostics and therapeutics to medical procedures and behavioral changes. This definition is intentionally holistic with regard to directionality, stage of intervention, development, and modality. Biomedical translation is not a one-step event, but multistep and recursive. “Observations” reflect that translation starts with the initial perception of phenomena, which must be demonstrated to be reproducible and robust. “Laboratory, clinic, and community” reflects the idea that translation need not start with a basic science observation that subsequently moves towards the clinic and ultimately public health; in fact, until relatively recently, most successful translations began with a clinical or public health observation that led to basic discoveries. Thus, translation is bidirectional. The bidirectionality of translation is particularly significant in translational science. With regards to “interventions”, translation is modality-agnostic; the translational process is conceptually similar whether its intended result is a small-molecule drug, a biologic, a device, a medical or surgical procedure, or a behavioral change such as diet, exercise, or smoking cessation. Thus, “translation” refers to a conceptual and practical multistep process. By contrast, “translational research” is defined by NCATS as the endeavor to traverse a particular step of the translation process for a particular target or disease. In the process from discoveries to medical applications in real-world environments, complexity as well as research and operational challenges increase exponentially.
Translational science is quite distinct in purpose and operation from translational research. Translational research focuses on the specific case of a target or disease, whereas translational science is focused on a general case that applies to any target or disease. Focus areas of translational science are the common causes of inefficiency and failure in translational research, such as incorrect predictions of the toxicity or efficacy of new drugs, lack of data interoperability, and ineffective clinical trial recruitment. Because these causes are the same across targets, diseases, and therapeutic areas, advances in translational science will increase the efficacy and safety of translational research in all diagnostic, therapeutic, and behavioral applications.
Like any other science, translational science seeks to elucidate general operative principles in order to transform translation from an empirical phenomenological process into a predictive science. Thus, translational science is clearly a nascent field. For historical and cultural reasons, translation has traditionally been practiced as an empirical craft, rather than studied as a science. The limits of empiricism in translation are evident in its persistently high failure rate and cost, which have continued to increase despite enormous efforts using the empirical paradigm. In this context, a thought experiment and a counterfactual are useful. The thought experiment is to imagine what drug development would be like if the general principles of small molecule target interactions were known, such that the activity of any compound on any target could be predicted effectively a priori. Failures due to unanticipated toxicity and lack of efficacy would decrease by orders of magnitude.
Like any other science, translational science will advance via research, i.e., translational science research that seeks to develop an understanding, technology, theoretical principle, or paradigm that will make the development of any therapeutic intervention more efficient and effective. The aggregate study of the results of individual translational research projects is one approach to elucidating translational science principles; conversely, individual translational research projects validate translational science principles and lead to their progressive advancement.
The relationship among basic research, translational research, and clinical research is expressed schematically in Fig. 4. Translational research consists of basic or preclinical research and clinical research. Much translational research is preclinical, from target validation to filing of an investigational new drug application. However, a great deal of clinical research is not translational, but rather focused on advancing the fundamental understanding of human physiology and pathophysiology. The intent of all basic research in the biomedical field is to understand the normal structure and function of living organisms, along with the characteristics and causes of abnormal structure and function, that is, disease. By contrast, the intent of translational research is to ameliorate, via physical or behavioral intervention, the abnormal structures and functions of an organism that are causing, or may lead to, disease. Translational science is among the newest sciences, emerging about 30 years ago to transform science and medicine.1)–3)
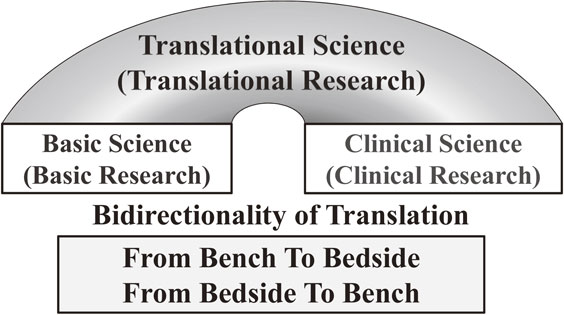
Schematic presentation of translational science, bridging basic science and clinical science. Bidirectionality, from bench to bedside, and, from bedside to bench, is essential in translational science. The significance of bidirectionality in translational research termed “bidirectionality of translation” is noted here.
The translational science spectrum represents each stage of research along the path from the biological basis of health and disease to interventions that improve the health of individuals and the public. The spectrum is not linear or unidirectional; each stage builds upon and informs the others. Patient involvement is a critical feature of all stages in translation.6)
2-2-1. Basic research.Basic research involves scientific exploration that can reveal fundamental mechanisms of biology, disease, or behavior. Each stage of the translational spectrum builds upon and informs basic research.
2-2-2. Preclinical research.Preclinical research connects basic research and human medicine. During this stage, scientists apply fundamental discoveries made in the laboratory or the clinic to further understand the basis of a disease or disorder, and then find ways to treat it. Hypothesis testing is carried out using cell or animal models, samples of human or animal tissues, or computer-assisted simulations of drugs, devices, or diagnostic interactions within living systems.
2-2-3. Clinical research.Clinical research includes clinical trials with human subjects to test intervention safety and effectiveness, behavioral and observational studies, outcomes and health services research, and testing and refining new technologies. The goal of many clinical trials is to obtain regulatory approval for an intervention.
2-2-4. Clinical implementation.The clinical implementation stage of translation involves the adoption of interventions into routine clinical care for the general population. This stage also includes implementation research to evaluate clinical trial results and identify new clinical questions and gaps in care.
2-2-5. Public health.In this stage of translation, researchers study health outcomes at the population level to determine the effects of diseases and efforts to prevent, diagnose, and treat them. Findings help guide scientists working to improve interventions or develop new ones.
2-2-6. Translational blocks.Two obstacles or translational blocks that impede efforts to apply science to improve human health in an expeditious fashion have been identified.1) The first block involves the transfer of new understandings of disease mechanisms gained in the laboratory into the development of new methods for diagnosis, therapy, and prevention, as well as their initial testing in humans. The second block affects the translation of results from clinical studies into everyday clinical practice and healthcare decision making. A systematic approach to addressing these two translational blocks have broad positive effects on health overall. At each juncture, along the continuum from basic biomedical research to clinical research and improved health, it is imperative that national clinical research enterprises have adequate resources infrastructure. The improved health that the public expects in turn for its investment in clinical research depends on clinical and medical coverage policy decisions which will allow the fruits of this research to reach every member of society.
The clinical research environment is itself part of the problem. Increasingly encumbered by rising costs, prolonged duration to results, inadequate funding, mounting regulatory burdens, fragmented infrastructure, incompatible databases, and a shortage of both qualified investigators and willing study participants.1) Essentially, the same situation confronts translational research in many developed countries, including Japan.
The discovery and purification of hormones in the 20th century are summarized in Fig. 5. In the first half of the 20th century, the sites of hormone production were thought to be the classical endocrine organs, or glands. However, in the latter half of the 20th century, it became clear that almost all organs in the body, including the cardiovascular system and adipose tissue, produce hormones. In addition to endocrine signaling, hormone signals are also transmitted in a paracrine or autocrine manner. A wide range of diseases are associated with hormone excess or deficiency, signal transduction abnormalities, and hormone resistance, involving the receptor and post-receptor molecules. Negative feedback systems, which form a general principle of endocrine regulation, maintaining homeostasis in the whole body. Hormones are designated as endogenous, intercellular, signal-transducing chemical substances. The toxic effects of hormones can be essentially ruled out when their levels are within the physiological range. These general principles in endocrinology and metabolism promoted diagnostic and therapeutic applications shortly after the discovery of hormones. The first hormone to be purified, synthesized, and applied to humans was adrenaline, which was purified by Dr. Jokichi Takamine as a cardiotonic or hypertensive substance,7) although it was often used as a hemostatic drug. Its agonists and antagonists are widely applied to treating not only endocrine diseases but also cardiovascular, respiratory, and urological diseases. Thus, it should be kept in mind that the goals of clinical application go beyond just validation of the original target disease.

Discovery and purification of hormones in the 20th century. After the discovery and purification of hormones in the 20th century, these discoveries have been applied rapidly to clinical practice for diagnostics and therapeutics.
The natriuretic peptide family consists of three structurally related peptides: ANP, BNP, and CNP. ANP has been identified and isolated from rat and human hearts, whereas BNP and CNP were isolated from porcine brain.8) The biological actions of natriuretic peptides are mediated by the activation of two subtypes of membranous guanylyl cyclase (GC), GC-A and GC-B, leading to intracellular accumulation of cyclic guanosine monophosphate (cGMP) (Fig. 6).9) The rank order of potency of induction of cGMP production via GC-A is ANP ≥ BNP ≫ CNP; for induction; via GC-B, the order, is CNP > ANP ≥ BNP.10) Thus, ANP and BNP serve as endogenous ligands for GC-A, whereas CNP is specific for GC-B. A third natriuretic peptide receptor with no intracellular GC domain, dubbed the clearance receptor (C-receptor), is thought to be engaged in the receptor-mediated degradation of natriuretic peptides (Fig. 6).9)

Schematic presentation of the natriuretic peptide system, consisting of ANP, BNP, and CNP, and their receptors, GC-A, GC-B, and clearance receptor. The intracellular second messenger is cGMP.
The primary structures of ANP, BNP, and CNP in various species are shown in Fig. 7. The primary amino acid sequence of ANP is highly conserved, whereas BNP differs markedly in molecular size and amino acid sequence among mammals.8) The structure of CNP, which has 22 amino acids, is identical in all known mammals (Fig. 7).

Primary structures of ANP, BNP, and CNP in various species. BNP exhibits a marked difference in molecular size and amino acid sequence, whereas the structure of CNP is identical in all known mammals.
The ANP/BNP/GC-A system plays pivotal roles in the regulation of cardiovascular homeostasis (Fig. 8), as demonstrated by its augmentation in various pathophysiological states such as heart failure,11)–15) myocardial infarction,16),17) cardiac hypertrophy,18),19) and hypertension.20)–22) ANP and BNP are cardiac hormones secreted primarily by the atrium and ventricle of the heart, respectively,15),22) and they have strong diuretic, natriuretic, and vasodilatory activities.11),12),15) Hence, ANP and BNP serve as sensitive biochemical markers for heart failure or cardiac hypertrophy (Fig. 9).13)–15) In particular, determination of plasma BNP levels has become a criterion for these conditions in routine clinical practice.23) ANP and BNP are both used in the treatment of heart failure.24),25) ANP infusion therapy (Suntory) was first approved as a drug for acute congestive heart failure by Pharmaceuticals and Medical Devices Agency (PMDA) in Japan (Fig. 10A), and subsequently, BNP injection therapy was approved by the U.S. Food and Drug Administration (FDA). Currently, ANP infusion therapy has reached >30% market share among the drugs given for acute congestive heart failure in Japan (Fig. 10B).

Physiological functions of the natriuretic peptide system as an endocrine, paracrine, and autocrine system in the peripheral organs. The natriuretic peptide system is also present in the central nervous system.

Diagnostic application of ANP and BNP. A: Schematic presentation of an immune-radio-metric assay for BNP. B: Plasma ANP and BNP concentrations in patients with congestive heart failure.

Therapeutic application of ANP and BNP. A: Intravenous drip infusion of ANP improves hemodynamic parameters in patients with congestive heart failure. B: Market share of heart failure drugs in Japan.
The posttranslational processing of the human BNP precursor is affected by its O-glycosylation,26) and plasma ProBNP levels change in human failing hearts,27)–30) as well as during renal failure.27),31)
The ANP/BNP/GC-A system in the heart inhibits angiotensin II type 2 receptor-mediated pro-hypertrophic signaling32) and also exerts anti-hypertrophic effects by inhibiting TRPC6 channel activity.33) The ANP/BNP/GC-A system in the kidney protects podocytes from aldosterone-induced glomerular injury34) and counteracts glomerular injury evoked by aldosterone through p38 mitogen-activated protein kinase inhibition.35) The ANP/BNP/GC-A system promotes muscle mitochondrial biogenesis and prevents obesity36) and inhibits hepatic damage and liver fibrosis.37)
CNP, the third member of natriuretic peptide family, was first purified from porcine brain.38) The structure of CNP is identical in all known mammals (Fig. 7). CNP-53, an endogenous high-molecular weight form of CNP, was also isolated from the brain.8) The half-life of CNP-53 is greater than that of CNP.8) Although CNP is the primary natriuretic peptide in the human brain,8),39) it is also produced by vascular endothelial cells40)–42) and macrophages.43) This hormone regulates vascular endothelial function and arteriosclerosis via local effects, rather than by acting as a circulating hormone in peripheral organs.8),44)–46) Substantial amounts of CNP and GC-B are expressed in the vascular wall, strongly suggesting the existence of a “vascular natriuretic peptide system” (Fig. 8).8),9),39),42),44) The CNP/GC-B system in the vasculature is implicated in the regulation of blood pressure and, vascular remodeling and regeneration, implicating it in hypertension and arteriosclerosis.8),9),47),48)
In bone, the CNP/GC-B system stimulates endochondral bone formation,2),49)–53) and defects in this system cause dwarfism2),49),51) with abnormal craniofacial growth.54) The CNP/GC-B system in the bone is also involved in the healing of bone fractures.55)
The distribution of the natriuretic peptide system overlaps with the distribution of the tissue reninangiotensin system,8),9),56)–58) prompting us to examine the functional relationship between the two (Fig. 11A). We revealed an antagonistic relationship between these two systems,8),9) both in their peripheral and central actions (Fig. 11B).8),9),59)–65) Furthermore, the natriuretic peptide system has therapeutic implications for vascular regeneration in patients with arteriosclerosis obliterans.48)

Regional distribution and function of the natriuretic peptide system (NPS). A: Regional distribution of the natriuretic peptide system in the human body. B: Antagonistic relationship of the natriuretic peptide system and renin–angiotensin system in the peripheral actions.
Because of the markedly short stature of CNP-KO mice, we analyzed food intake and energy expenditure of mice generated by crossing CNP-KO mice with a strain with chondrocyte-targeted CNP expression; this rescued marked skeletal dysplasia, allowing us to investigate the significance of CNP with minimal influence of skeletal phenotypes. This study unveiled a new role for CNP in regulating food intake and energy expenditure.66) In addition, we reported that intracerebroventricular administration of CNP suppresses food intake via activation of the melanocortin system in the hypothalamus in mice.67) We also demonstrated that brain-specific GC-B deletion attenuated high fat-induced visceral and hepatic lipid deposition in mice.68) These results clearly indicate that the CNP/GC-B system, as a neuropeptide in the brain, is involved in negative regulation of food intake and energy expenditure.
3-2. Mice with genetic alterations in the ANP/BNP/GC-A system.Genetically engineered mice are useful tools for studying the complex phenotypic effects of an altered gene in living animals. Mice harboring overexpression or deficiency of each member of the natriuretic peptide family, or its cognate receptor, have been generated through Tg or KO technologies.69)–73) We generated Tg mice expressing BNP under the control of the serum amyloid P (SAP) component promoter, which targets hormone expression to the liver.71) BNP-Tg mice exhibited a 100-fold increase in plasma BNP concentrations, with concomitant elevation of plasma cGMP concentrations. These mice had significantly lower blood pressure and smaller hearts than non-Tg littermates.71) These results indicated that BNP functions in long-term cardiovascular regulation and may be useful as a long-term therapeutic agent. In addition, proteinuria and renal dysfunction observed in anti-glomerular basement membrane nephritis,74) nephrosclerosis induced by subtotal nephrectomy,75) and manifestations of diabetic nephropathy76) were ameliorated in BNP-Tg mice relative to those in wild-type mice, indicating a possible application of GC-A agonists, ANP or BNP, in the treatment of renal diseases.
We also generated mice bearing targeted disruption of the BNP gene.72) At baseline, BNP-KO mice did not exhibit any signs of systemic hypertension or ventricular hypertrophy. However, these animals developed multifocal fibrotic lesions within the cardiac ventricle even in the absence of additional stresses; these lesions increased in size and number in response to ventricular pressure overload, demonstrating that BNP is an anti-fibrotic factor acting within the ventricle of the heart as an autocrine/paracrine regulator to promote ventricular remodeling.72) In addition to these cardiovascular manifestations, BNP-Tg mice exhibited marked skeletal overgrowth via endochondral bone formation.77) Nevertheless, BNP-KO mice did not possess any skeletal abnormalities.72) The skeletal overgrowth seen in BNP-Tg mice expressing elevated plasma concentrations of BNP was similar to that seen in cartilage-specific CNP-Tg mice.78) Given that mice with genetic disruption of the ANP/BNP/GC-A system do not have any abnormal skeletal phenotypes,72),79) we postulated that the markedly increased circulating levels of BNP (100-fold relative to wild-type mice) may cross-react with GC-B to stimulate endochondral bone growth, even though the affinity of BNP for GC-B is lower than that for GC-A.9) This interpretation was supported by the finding that the skeletal overgrowth observed in BNP-Tg mice was not abrogated by a genetic deficiency of GC-A in BNP-Tg mice.78)
ANP-Tg mice, which express elevated levels of circulating ANP under the control of a mouse transthyretin promoter, exhibited decreased arterial blood pressure without the induction of diuresis or natriuresis.69) ANP-KO mice and GC-A-KO mice exhibited salt-sensitive70) and salt-resistant hypertension,73) respectively. Studies of GC-A-KO mice implicated GC-A in anti-hypertrophic actions in the heart.79)–84) A more detailed analysis of GC-A performed using conditional KO mice indicated the importance of GC-A in vascular endothelial cell-mediated blood pressure regulation.82)–84)
As for the regulation of ANP and BNP gene expression, neuron-restrictive silencer elements (NRSEs) are located in the 5′-flanking region of the BNP gene and the 3′-untranslated region of the ANP gene.85) Neuron-restrictive silencer factor (NRSF) can thus repress ANP promoter activity through binding to NRSE.86) Studies examining mice expressing dominant-negative NRSF-Tg under the control of an alpha-myosin heavy-chain promoter have demonstrated that NRSF plays an important role in the gene expression of ANP and BNP, as well as in the progression of cardiac dysfunction and lethal arrhythmia associated with heart failure.87) We also crossed dominant-negative NRSF-Tg mice with GC-A-KO mice to assess the effects of endogenously expressed ANP and BNP during the progression of cardiomyopathy, demonstrating that endogenous ANP and BNP protect the heart against cardiomyocyte death and the progression of pathological remodeling in a mouse model of dilated cardiomyopathy and sudden death.88) Myocardin-related transcription factor-A is a common mediator of mechanical stress and neurohumoral stimulation-induced cardiac hypertrophic signaling leading to activation of BNP gene expression.89)
3-3. Genetically engineered mice and rats of the CNP/GC-B system.We generated mice with a targeted disruption of the CNP gene; the resultant CNP-KO mice exhibited markedly short stature due to impaired bone growth.49) Mammalian bones are formed through two different mechanisms, endochondral ossification and membranous ossification. Most mammalian bones are formed through endochondral ossification, during which chondrocytes in the growth plate undergo proliferation, hypertrophy, cell death, and osteoblastic replacement.50)
The short stature phenotype of CNP-KO mice resulted from impairment of bone growth through endochondral ossification.49) CNP-Tg mice with targeted overexpression of CNP in the growth plate cartilage exhibited prominent overgrowth of bones formed through endochondral ossification.49) GC-B-KO mice exhibited the same short stature phenotype90) as CNP-KO mice,49) demonstrating that the CNP/GC-B system is a physiologically important stimulator of endochondral bone growth. Dominant-negative GC-B-Tg rats exhibited blood pressure-independent cardiac hypertrophy, suggesting that GC-B signaling is linked to the control of cardiac growth.91)
cGMP dependent protein kinase (cGK) has been identified as a molecule activated downstream of the natriuretic peptide family and GC system.92) Mice with a gene depletion of one subtype of cGK, cGKII-KO mice, have a short stature phenotype secondary to impaired endochondral bone growth,93) similar to the phenotype of CNP-KO mice.49) We demonstrated that cGKII affected endochondral bone growth by functioning downstream of the CNP/GC-B system, by showing that impaired endochondral bone growth observed in cGKII-KO mice could not be rescued by targeted overexpression of CNP in the growth plate cartilage.94)
Multiple spontaneous animal models with impairments in the CNP/GC-B system have been identified.95)–98) Two strains of dwarf mice, with autosomal recessive mutant genes, named cn/cn95) and short-limbed dwarfism (SLW),96) have spontaneous loss-of-function mutations in the GC-B gene. Spontaneous mutant mice with a loss-of-function mutation in the CNP gene, named long bone abnormality (lbab) mice, exhibit short stature owing to impaired endochondral bone growth,98) and this phenotype can be abrogated by targeted overexpression of CNP in the growth plate cartilage.99)
Cartilage-specific CNP-KO mice100) have a defect in endochondral bone very similar to that of phenotype of systemic CNP-KO mice.49) Cartilage-specific KO of the GC-B gene100) also results in an essentially identical phenotype to that of systemic KO of CNP,90) whereas the disorder of endochondral bone formation in cartilage-specific GC-B-KO mice is more severe than that of cartilage-specific CNP-KO mice.100)
The blood pressure of endothelium-specific CNP-KO mice is significantly higher than that of wild-type mice, whereas the blood pressure of smooth muscle cell-specific GC-B-KO mice did not differ from that of wild-type mice.47) This finding indicates that GC-B in vascular endothelial cells, rather than vascular smooth muscle cells, is responsible for the control of blood pressure.
To rule out species-related differences in the physiological function of the CNP/GC-B system, we generated systemic CNP-KO rats.101) We confirmed an identical short stature phenotype due to defective endochondral bone formation in CNP-KO rats, although unlike the mouse CNP-KO model, the knockout rats did not die early.49) Studies are ongoing to clarify the causes and mechanism underlying the species-related difference underlying early death. It might be explained in part by craniofacial deformities, including defects in the foramen magna, in CNP-KO mice, because such abnormalities can induce neurological damage.54),102)
3-4. Clinical application of CNP and its analogs for skeletal dysplasia.To explore the potential clinical applications of CNP and its analogs, we tested the strong effect of the CNP/GC-B system on endochondral bone growth in the context of skeletal dysplasia, a group of genetic disorders characterized by severely impaired bone growth.103) Achondroplasia (Ach), the most common form of skeletal dysplasia, is characterized by short-limbed dwarfism, (Fig. 12A) and is caused by a constitutively active mutation in fibroblast growth factor (FGF) receptor 3 (FGFR3).104) Current therapy for Ach is limited to distraction osteogenesis,105) an orthopedic procedure; no efficient drug therapies have yet been developed.

Clinical implication of the CNP/GC-B system in achondroplasia (Ach). A: A human patient with achondroplasia with a constitutively active mutation of FGFR3. B: Effect of CNP on body length, based on mating of achondroplasia model mice a with CNP-Tg mice. C: Molecular mechanism of the therapeutic effect of CNP in achondroplasia.
We demonstrated that targeted overexpression of a CNP transgene in the growth plate cartilage of a mouse model of Ach rescues their impaired bone growth and short stature phenotype (Fig. 12B).51) To elucidate the molecular mechanism by which CNP ameliorates Ach, we examined the effects of CNP on extracellular signal-regulated kinase (ERK) signaling. CNP inhibited FGF2-stimulated phosphorylation of ERK in a dose-dependent manner through cGMP activation via GC-B (Fig. 12C), ultimately increasing matrix synthesis by chondrocytes.51)
To determine whether chronically elevated plasma CNP reaches the growth plate and stimulates endochondral bone formation, we generated CNP-Tg mice that overexpressed CNP in the liver. Circulating CNP stimulated skeletal growth,106),107) indicating that CNP injection therapy may be an effective treatment.
We also demonstrated that systemic and continuous administration of synthetic CNP is an effective and safe way to reverse the impaired bone growth of Ach mice (Fig. 13).108) The efficacy and safety of systemic CNP administration in preclinical studies,108) together with the observation that intravenously injected CNP has only a minimal effect on blood pressure in humans,109) implies that the systemic administration of CNP or CNP analogs represents a novel therapeutic strategy for treating human skeletal dysplasia, including Ach. One form of human skeletal dysplasia, acromesomelic dysplasia type Maroteaux, is caused by loss-of-function mutations in the GC-B gene,110) which cannot be treated with CNP or CNP analogs.110) These results clearly indicate that the CNP/GC-B system is a physiologically important enhancer of endochondral bone growth in humans, suggesting that CNP and CNP analogs could be applied to multiple types of human skeletal dysplasia.2),51)–53)

Therapeutic effect of intravenous infusion of synthetic CNP on achondroplasia (Ach) model mice. A: Gross appearance of achondroplasia model mice treated with CNP by intravenous infusion. B: Growth curves of achondroplasia model mice treated with CNP by intravenous infusion. The growth of CNP-treated achondroplasia model mice caught up with that of wild-type (Wt) mice.
A clinical trial of a neutral endopeptidase-resistant CNP analog, aimed at receiving FDA approval, is now underway in multiple countries including Japan.111)
3-5. Clinical application of CNP and CNP analogs for diseases other than skeletal dysplasia.Idiopathic short stature, a common disease of short stature phenotype with an unknown etiology, and bone fracture that heals through endochondral ossification,55) are the next likely avenues in which a therapeutic effect of CNP and its analogs might be discovered. In addition, CNP and CNP analogs will be useful for treating vascular remodeling and arteriosclerosis via vascular endothelial cells and macrophages, as well as chronic inflammation via macrophages.2),47),48) Because the CNP/GC-B system is involved in the negative regulation in food intake and energy expenditure, non-peptide agonists for GC-B may be used for the treatment of obesity, diabetes mellitus, and steatohepatitis.36),37),66)–68)
Leptin, an adipocyte-derived hormone, has been demonstrated to cause an overt obese phenotype upon deletion in both animals and humans. Leptin was originally identified as a hormone absent from hereditary obese (ob/ob) mice.112) This hormone plays crucial physiological roles in the regulation of energy expenditure and food intake.113)–117) Mice118) and rats119) harboring mutations in leptin receptors exhibit phenotypes identical to those of ob/ob mice. The Koletsky rat, an obese sub-strain of SHR that serves as a model of metabolic syndrome with both hypertension and morbid obesity, carries an additional nonsense mutation in the leptin receptor.120)
In obese animals and patients, plasma or serum leptin concentrations are increased in proportion to the degree of adiposity,121)–123) indicating that leptin is a satiety signal communicating the size of adipose stores to the brain,124)–126) and that leptin resistance is related to obesity.121),127)–129) Leptin deficiency in human patients is associated with morbid obesity and insulin resistance, indicating that leptin plays a physiological role in both animal models and humans.130),131) Leptin is implicated in a number of phenotypes observed in obese animal models,125),132)–135) especially obesity-related hypertension,133) abnormal reproduction,132) bone changes,134) and Cushing's syndrome.136)
Figure 14 compares the phenotypes of obesity and generalized lipodystrophy. The two diseases have similar phenotypes related to glucose and lipid metabolism, although there is a striking contrast between them in adiposity and serum adipokine levels: too high in obesity vs. almost absent in generalized lipodystrophy. This underscores the importance of maintaining normal adiposity in order to preserve health.2),137),138)
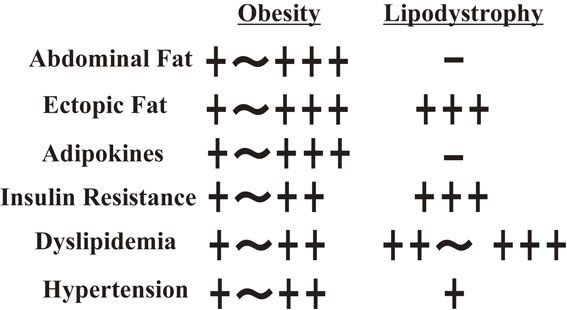
Comparison of phenotypes between obesity and generalized lipodystrophy. The two diseases exhibit similar phenotypes in glucose and lipid metabolism, although there is a striking contrast between them in adiposity and levels of adipocyte-derived serum adipokines levels.
Leptin is also produced by the human placenta139) and choriodecidual tumors.140) The functional significance of leptin in pregnancy and tumors remains unclear.
4-1. Generation of Tg mice overexpressing leptin.To explore the clinical implications of leptin in vivo, we generated leptin-Tg “skinny” mice with elevated plasma leptin concentrations similar to those in obese humans.141) A fusion gene consisting of the human SAP promoter upstream of the mouse leptin cDNA coding sequence was designed in order to target hormone expression to the liver.142) Overexpression of leptin in the liver resulted in complete disappearance of both white and brown adipose tissues in mice.141) However, this phenotype did not occur when transgene expression was targeted to adipose tissue, the endogenous site of leptin production, using adipocyte-specific promoters.143) The hyperleptinemia seen in these transgenic “skinny” mice provides a unique experimental system, in which the long-term effects of leptin can be investigated.132)–135),141),144)–146) Skinny mice exhibit augmented glucose metabolism and increased insulin sensitivity in both skeletal muscle and liver,141) supporting the idea that leptin acts as an anti-diabetic hormone in vivo.147)–149) These studies suggest that leptin could be useful treating diabetes and obesity.
4-2. Crossbreeding of transgenic skinny mice with A-ZIP/F-1 mice, a mouse model of severe lipoatrophic diabetes.Generalized lipodystrophy with a systemic deficiency of adipose tissue, is characterized by severe insulin resistance and hypertriglyceridemia.150) A form of diabetes called lipoatrophic diabetes, eventually develops, although the precise mechanism by which this paucity of fat results in diabetes remains to be elucidated. Plasma leptin concentrations are markedly reduced or absent in patients with lipoatrophic diabetes, as well as in rodent models of this disease.151)–154) Given the antidiabetic action of leptin, its deficiency may play a role in the pathogenesis of lipoatrophic diabetes; thus, leptin may be useful as a drug to treat lipoatrophic diabetes.
A mouse model of severe lipoatrophic diabetes (A-ZIP/F-1) was generated, by expressing a protein that inactivates basic-zipper transcription factors in adipose tissue.152) To assess the pathophysiological role and therapeutic potential of leptin in lipoatrophic diabetes, we crossed transgenic skinny (leptin Tg/+) and A-ZIP/F-1 (A-ZIP Tg/+) mice to yield double-transgenic mice (leptin Tg/+: A-ZIP Tg/+) that lack adipose tissue and express approximately 10-fold higher leptin levels than controls (Fig. 15A).155) Leptin Tg/+: A-ZIP Tg/+ mice were hypophagic relative to A-ZIP Tg/+ mice and exhibited decreased hepatic steatosis. Glucose and insulin tolerance tests revealed increased insulin sensitivity and normal glucose tolerance in leptin Tg/+: A-ZIP Tg/+ mice, similar to those in leptin Tg/+ mice. Pair-feeding experiments demonstrated that the effects of leptin were not solely due to decreased food intake. Leptin also helped to prevent diabetic nephropathy in generalized lipoatrophic diabetes mice.135) These results demonstrated that leptin can improve insulin resistance and diabetic manifestations in a mouse model of severe systemic lipodystrophy, indicating that leptin is therapeutically useful in the treatment of lipoatrophic diabetes.155)

Phenotypic effects of cross-mating of generalized lipodystrophy model mice (A-ZIP Tg/+) with leptin-Tg skinny mice (Lep Tg/+). A: Therapeutic effects of leptin on plasma glucose levels in generalized lipodystrophy model mice. B: Therapeutic effects of leptin on plasma levels of insulin in generalized lipodystrophy model mice. Doubly transgenic mice (Lep/A-ZIP) had normal glucose metabolism and levels of insulin.
We previously reported a novel homozygous mutation of the melanocortin 4 receptor (MC4R) in a Japanese woman with severe obesity (body mass index [BMI] 62 kg/m2).156) MC4R mutations have been identified in morbidly obese patients in Europe at a relatively high frequency (3–4%); all of the mutations reported to date occur in an autosomal-dominant fashion, with the exception of a single unique pedigree in the U.K.157),158) Although both parents were heterozygous for the mutation, neither exhibited such a severe obese phenotype (BMI 27 kg/m2 and 26 kg/m2, pre-obese according to WHO criteria and, grade 1 obesity in Japan). Given that genetic backgrounds and lifestyle vary significantly between European and Asian countries, it is necessary to examine the effect of lifestyle on phenotypes resulting from genetic mutations, as well as treatment efficacy, in each country.
The first case for leptin replacement therapy with metreleptin (Shionogi in Japan) was an 11-year-old girl with acquired generalized lipodystrophy, seen 6 months after onset (Fig. 16A).159),160) Leptin replacement therapy dramatically normalized fasting glucose levels within 1 week (Fig. 16B), and also normalized the 75 g oral glucose tolerance test and HbA1c within 3 months (Fig. 16C). The dramatic effects of leptin replacement therapy on glucose and lipid metabolism without any ill effects in the first case accelerated clinical studies in Japan.

Dramatic effects of leptin replacement therapy on glucose metabolism in the first case, an 11-year-old girl with acquired generalized lipodystrophy. A: Pictures before and 1 year after onset. B: Fasting plasma glucose normalized within 1 week of starting leptin replacement therapy. C: Oral glucose tolerance test (75 g) and HbA1c concentration also normalized within 3 months.
Four months of leptin replacement therapy was reported to improve glucose and lipid metabolism in lipodystrophy patients in the U.S.161) To elucidate the efficacy, safety, and mechanisms underlying leptin replacement therapy in Asian patients with generalized lipodystrophy, we treated seven Japanese patients, two acquired and five congenital, with a physiological replacement dose of leptin (Fig. 17).159),160) Leptin-replacement therapy significantly improved fasting glucose (mean ± SE, 172 ± 20 to 120 ± 12 mg/dl; P < 0.05) (Fig. 17A), and triglyceride (mean ± SE, 700 ± 272 to 260 ± 98 mg/dl; P < 0.05) levels (Fig. 17C) within 1 week and normalized HbA1c within 2 months (Fig. 17B). Leptin replacement therapy decreased insulin resistance, as demonstrated by the euglycemic clamp method (Fig. 18). Improvement in fatty liver was also confirmed by changes in computed tomography (CT) attenuation, and reduction of liver volume was confirmed by CT imaging (Fig. 17D). By 4 months, six of seven patients were able to discontinue all antidiabetic drugs, including insulin. The reductions in fasting plasma glucose levels, HbA1c, triglyceride levels, and liver volumes in all seven patients were maintained throughout the therapeutic period with no adverse effects (Fig. 17). The longest period of leptin replacement therapy had extended beyond 7 years,160) and has now reached 18 years for the first case. Functional magnetic resonance imaging analysis clearly demonstrated changes in food-related brain activity in patients with generalized lipodystrophy before vs. after leptin replacement therapy.162)

Chronic leptin replacement therapy improved fasting plasma glucose levels (A), HbA1c (B), plasma triglyceride levels (C), and liver volumes (D).

Leptin replacement therapy gradually improved insulin sensitivity in patients within 4 months examined using a monthly glucose clamp test.
Leptin replacement therapy was also effective at combating diabetic complications.2),160) The macroalbuminuria seen in two patients regressed to microalbuminuria, whereas microalbuminuria in two other patients normalized. The creatinine clearance of patients with glomerular hyperfiltration decreased, concomitant, with improved glucose tolerance (Fig. 19), consistent with previous findings in mice models of lipoatrophic diabetes (Fig. 20).135)

Leptin replacement therapy improved microalbuminuria and also improved the glomerular hyperfiltration rate in a patient with diabetic nephropathy complicated with generalized lipodystrophy.

Schematic presentation of the effects of leptin replacement therapy in generalized lipodystrophy model mice on prevention for diabetic nephropathy in mice mated with leptin-Tg skinny mice, and its amelioration after onset of diabetic nephropathy.
We also examined the effects of the leptin replacement therapy in a 16-year-old girl with severe hypertriglyceridemia (>1,000 mg/ml; approximately 8,000 mg/ml at the peak) who suffered from repeated episodes of acute pancreatitis. After the initiation of leptin therapy, her triglyceride levels normalized and she did not have any additional episodes of acute pancreatitis.2),160) These results clearly demonstrate the striking efficacy and safety of long-term leptin-replacement therapy in patients with generalized lipodystrophy.2),160) Although these results are impressive, it is important to remember that the efficacy of leptin-replacement therapy appears to be much higher in Japan, a country where the prevalence of obesity is relatively low, than in countries with a high prevalence of obesity.2),160) The change in satiety sense due to the leptin replacement therapy could be recognized within a few days in adult patients, although it was less clear in children.159),160)
Chronic leptin replacement therapy for generalized lipodystrophy patients induced no significant changes in systolic and diastolic blood pressures (Fig. 21), or bone mineral density (Fig. 22). Because the generalized lipodystrophy patients examined in clinical research were younger than 50 years, the effects of chronic leptin replacement therapy on blood pressure and bone mineral density should be followed up carefully.

Chronic leptin replacement therapy did not induce any significant changes in systolic or diastolic blood pressure in patients with generalized lipodystrophy.

Chronic leptin replacement therapy did not induce any significant changes in bone mineral density in patients with generalized lipodystrophy.
Leptin therapy for partial lipodystrophy has been approved only in Japan.163) The first use of leptin therapy for partial lipodystrophy, a 28-year-old woman (BMI: 21.3 kg/m2, leptin: 2.1 ng/ml) with familial partial lipodystrophy, was effective and safe for more than 10 years (Fig. 23); recently, the patient was able to have a baby, which she had strongly desired. In addition, patients with atypical partial lipodystrophy have been reported in Japan, as well as in other countries.164)–166) Leptin therapy was also effective and safe in two cases of partial lipodystrophy (Case 1, BMI: 16.9 kg/m2, leptin: 6.5 ng/ml, Case 2, BMI: 17.1 kg/m2, leptin: 3.5 ng/ml) in Japan,166) suggesting that the efficacy of leptin therapy in partial lipodystrophy depends on BMI and plasma leptin concentrations. BMI and plasma leptin concentrations are known to reflect the degree of adiposity.121)–123) A highly sensitive leptin assay method (Cosmic Corporation) was recently approved as a diagnostic for lipodystrophy by PMDA in Japan (Table 1). No neutralizing antibodies were reported in these patients, but the appearance of neutralizing antibodies should be kept in mind as a possible consequence of chronic leptin administration with metreleptin in partial lipodystrophy.

Therapeutic effects of leptin therapy on partial lipodystrophy. A: MRI (T1) image of a 28-year-old female patient with familial partial lipodystrophy disclosed presence of the adipose tissue in the head region, bone marrow and sole and loss of the subcutaneous adipose tissue in both upper and lower extremities. B: Leptin therapy improved glucose tolerance, and HbA1c decreased from 6.7% to 5.3%, for 2 months in a patient with familial partial lipodystrophy. C: Lateral view of a patient with partial lipodystrophy like Cushing's syndrome.
To assess the therapeutic potential of leptin therapy in insulin-deficient diabetes, we generated diabetic animal models by treating wild-type and leptin-Tg mice with a relatively low dose of streptozotocin (STZ) (180 g/g body weight).167) Plasma insulin concentrations were reduced (<0.10 ng/ml), resulting in severe hyperglycemia in both wild-type and leptin-Tg mice 2 weeks after STZ treatment. Leptin-Tg mice were more sensitive to exogenously administered insulin than wild-type mice. STZ-treated leptin-Tg mice became normoglycemic at doses of insulin that did not improve hyperglycemia in STZ-treated wild-type mice. To determine whether combination therapy with leptin and insulin is beneficial for insulin-deficient diabetes, we also examined the effect of chronic co-administration of leptin and insulin in STZ-treated wild-type mice. The results revealed that sub-threshold doses of insulin that do not affect glucose homeostasis, in combination with leptin, are effective at improving diabetes in STZ-treated wild-type mice. We also demonstrated that leptin therapy is useful for the treatment of diabetic complications and increases longevity in insulin-deficient diabetic mice.168) These results indicate that leptin therapy may be used as an adjunct to insulin therapy in insulin-deficient diabetes.167),168)
We also investigated the therapeutic usefulness of leptin in a mouse model of type 2 diabetes mellitus with increased adiposity,169) generated using a combination of a low-dose (STZ120 g/g body weight) and a high-fat diet (HFD; 45% of energy as fat) (STZ/HFD). In STZ/HFD mice, continuous infusion of leptin (20 ng/g body weight/hour) reduced food intake and body weight gain, and also improved glucose and lipid metabolism which enhancing insulin sensitivity. Leptin therapy also decreased triglyceride content in both liver and skeletal muscle. These results indicate a beneficial effect of leptin therapy for type 2 diabetes mellitus with increased adiposity, which corresponds to BMI in the range of 2530 kg/m2, pre-obese according to WHO criteria or grade 1 obesity in Japan.169)
Our previous and ongoing studies on leptin-Tg skinny mice and other mouse animal models, have revealed pleiotropic actions of leptin in the regulation of energy homeostasis and food intake,132)–135),141),144),145),170) and suggested that leptin might be clinically useful as a therapy for a variety of diseases, particularly diabetes mellitus,144),155),159),160),168) depression with obesity,171) and steatohepatitis.172),173) Leptin-Tg skinny mouse could be a useful model for studying the long-term effects of leptin action in vivo and evaluating the clinical implications of the therapy.2)
To further assess the target validation of diabetes mellitus beyond species-specific differences, rat models for obesity and general lipodystrophy were established via N-ethyl-N-nitrosourea mutagenesis of the leptin gene174) and the seipin gene,175) respectively. Steatohepatitis in ob/ob mice was aggravated by pioglitazone and rosiglitazone treatment, but improved in leptin-KO obese rats, being consistent with the beneficial effects of these compounds on steatohepatitis in humans.174) Thus, mouse disease models are not always the suitable for studying human disease.
4-6. Drugs for leptin resistance.The prevalence of obesity and its associated lifestyle-related diseases, including metabolic syndrome, is increasing worldwide. Hyperleptinemia and central leptin resistance or insufficiency of leptin function are the main features of obesity, suggesting that leptin sensitizers represent promising target molecules for drug discovery and treatment of obesity. Amylin and glucagon-like peptide-1 (GLP-1) agonists are peptide-nature leptin sensitizers.176),177) The naturally occurring phytochemicals celastrol and withaferin A, both identified in thunder god vine, are leptin sensitizers.178)–180) Leptin-responsive cell lines from adult mouse hypothalamus are good tools for studying the molecular mechanism of leptin sensitizers.181)
ANP and BNP concentrations increase in parallel with the severity of congestive heart failure, suggesting a diagnostic and therapeutic application in the context of this disease.2),8),11)–15) The increase in plasma BNP concentration in patients with severe congestive heart failure is three orders of magnitude higher than normal plasma,2),8),13)–15) and the source of plasma BNP is the ventricle of the heart.2),8),13) Therefore, the measurement of plasma BNP concentration is an indispensable diagnostic method in clinical practice, corresponding to the “Public Health” step on the translational science spectrum. Because the half-life of plasma BNP is longer than that of ANP,2),8),13) BNP is administered by the bolus injection method in the U.S.A.; ANP is administered by the drip infusion method in Japan.24) For treatment of congestive heart failure2),8),24),25) and renal failure without unstable hemodynamic changes, drip infusion of ANP and BNP is much safer than bolus injection of BNP, which is impossible to adjust. Treatment of congestive heart failure by drip infusion of ANP, as performed in Japan corresponds to the “Clinical Implementation” step on the translational science spectrum.
The POC of CNP therapy for Ach is based on the observation that endochondral bone formation in the growth plate is disrupted in CNP-KO mice and accelerated in CNP-Tg mice.2),49) Disrupted endochondral bone formation in an Ach model mouse is improved by cross-mating with CNP-Tg mice or by CNP infusion.51)–53),107) CNP therapy for patients with Ach corresponds to the “Clinical Research” step on the translational science spectrum.
The POC of leptin-replacement therapy for patients with lipoatrophic diabetes or generalized and partial lipodystrophy is derived from basic and preclinical research using animal models, including mice2),138),155) and rats,174) which emulate human generalized lipodystrophy remarkably well. In addition, the serum leptin concentration is markedly reduced or almost absent in patients with generalized lipodystrophy, and low to normal in patients with partial lipodystrophy. Leptin replacement therapy is effective and safe for patients with generalized and partial lipodystrophy. Leptin replacement therapy was approved for the first time by the PMDA in 2014. Therefore, leptin replacement therapy corresponds to the “Clinical Implementation” step on the translational science spectrum.
In medicine or clinical practice, patients are genetically heterogeneous, whereas the experimental animals used in basic research and preclinical research are strains, i.e., genetically homogeneous. The differences in genetic background between disease model animals and human patients is critical for the evaluation and interpretation of results regarding physiological and pharmacological functions obtained from basic and clinical research. In basic research, lower animals such as yeast, C. elegans, Drosophila, and zebrafish are used as model animals. The distribution and primary structures of protein and peptide hormones, their receptors, and signal transduction molecules, as well as the final products of biosynthesis of non-protein, or non-peptide hormones, differ significantly between lower animals and humans. In particular, the primary structure and tissue distribution of BNP and posttranslational processing of the BNP precursor exhibit marked species-differences (Fig. 7).2),8),39) BNP was first discovered in the porcine brain,8),38) but very little BNP is detectable in the brains of rodents and humans.2),8),39) Thus, on the “Basic Research” and “Preclinical Research” steps of the translational science spectrum, translational scientists should carefully consider the possible existence of species-related differences, from the standpoints of both efficacy and safety.
In translational research on ANP as a drug for congestive heart failure,2),8),24) and on ANP and BNP as diagnostics for congestive heart failure,2),8),13)–15) the disease target of our translational research was a common disease, whereas the POC of CNP therapy was established for Ach, and that of leptin replacement therapy was established for patients with lipodystrophy, both of which are rare diseases with a very limited number of patients (Fig. 24). It is very likely that drugs that are efficacious and safe for the treatment of rare human diseases will provide us with novel treatments for common human diseases, which are adjacent to or extensions of rare human diseases in both etiology and pathophysiology. Lessons from the research history of SHRs as a useful animal model for essential hypertension, in which almost all antihypertensive drugs for human essential hypertension were known to be efficacious, and from the history of clinical application of blockade of the renin-angiotensin system originating from relatively rare hypertension, renovascular hypertension, to widespread cardiovascular diseases including essential hypertension, congestive heart failure and renal diseases2) should be kept in mind as concrete examples of translational research. Common features of translational research provide us with important principles for translational science via: 1) suitable disease animal models in the preclinical phase, 2) rare human diseases in the clinical phase of translational research, and 3) the subsequent widespread clinical applications to common human diseases, beyond the original target validation, that are adjacent to or extensions and share the mechanistic implications of rare human diseases.

Clinical applications, from rare diseases to common diseases, originating in translational research on CNP and leptin.
Selection and analysis of appropriate animal disease models emulating human diseases are essential for successful clinical applications. To this end, selection of suitable animal models must be based on multifaceted clinical experience and substantial knowledge of the target human diseases. The original research targets may well be rare diseases. More importantly, one can hope and try to expand the scope of the research into common diseases with the aid of “clinical wisdom”. Clinical wisdom may be derived from multiple bedside experiences and substantial knowledge in human physiology and pathophysiology.
Clinical investigators or physician-scientists (or clinician-scientists) who are familiar with both basic science and clinical science, which is bidirectional, should become authentic translational scientists. There is no question that close and precise collaboration among basic scientists, translational scientists, and clinical scientists is essential for advancing translational science, not only in Japan, but all over the world.
The author was born and grew up in a rural, doctorless village environment and enjoyed insect collecting and had a vague dream of becoming a medical doctor or scholar. A famous historical person from the author’s hometown, Mr. Kunimichi Kitagaki, was a patriot at the end of the Edo period, became the Governor of Kyoto, and founded the Lake Biwa Canal in 1890, which enabled Japan to build its first hydroelectric power plant. As a medical student at Kyoto University, the author visited the late Professor Osamu Hayaishi, a great biochemist, with my classmate (later, Professor Shu Narumiya, who became Professor, Department of Pharmacology, Kyoto University), and had the chance to be introduced to basic research on biochemistry. In his laboratory, during the summer vacation and after school, we learned the pleasure of basic research. We obtained valuable experience similar to that of students on an MD-PhD course, although there was no such course at that time in Japan. Later, after entering the Graduate Course of Internal Medicine at Kyoto University, headed by the outstanding endocrinologist Professor Hiroo Imura, my MD-PhD thesis work focused on the clinical significance of endogenous opioid peptides such as β-endorphin, enkephalins, and leumorphin.182)–188) My research into opioid peptides involved strict but excellent supervision from late Professor Shousaku Numa, a great molecular biologist. These fortune encounters with three excellent mentors in biochemistry, basic science, and internal medicine, and clinical science, respectively, enabled the author to become a bidirectional physician-scientist. The past 35 years, has seen my engagement in translational research on novel hormones, especially the natriuretic peptide family and leptin, as a translational scientist bridging basic or preclinical research and clinical application. My enthusiasm for the achievement of translational research and science might originate in part from my dream and respect in childhood.
The author’s research career has involved close collaboration with Professors Kenji Kangawa and Hisayuki Matsuo of Miyazaki Medical College (later of National Cardiovascular Research Center, Osaka), who isolated the natriuretic peptide family. Further collaborations have been with Professor Hirofumi Yasue and his colleagues at the Department of Cardiology, Kumamoto University Graduate School of Medicine, especially in the clinical applications of ANP and BNP. To promote research and the exchange of knowledge about preclinical and clinical studies on these cardiovascular hormones, we founded “The Society of Cardiovascular Endocrinology and Metabolism (CVEM)” in 1996. In addition to collaborating with the late Dr. Nobuo Yoshida of Shionogi on the diagnostic applications of ANP and BNP for congestive heart failure, working with young PhD staffs at Suntory (later, Daiichi-Sankyo) has developed ANP and BNP as drugs for congestive heart failure. Current collaborations with Chugai Pharmaceutical are looking into the clinical applications of CNP. Translational scientists should be familiar with scientists in the pharmaceutical industry and not miss chance encounters with those in both the academia and industry.
Medical applications of diagnostics, therapeutics, and behavioral changes, “needs” should be accomplished by clinical applications of discoveries “seeds”. Medical research is effort in vain without clinical application. As a translational physician-scientist, it is not possible to forget the personal sense of accomplishment that was felt when we succeeded in developing diagnostic and therapeutic applications of the natriuretic peptide family and leptin.
It must be hoped that young, talented medical students, including MD-PhD students, will seek to become translational physician-scientists, who might otherwise become an endangered species “bewitched, bothered, and bewildered-but still beloved”, as pointed out by Professors J. L. Goldstein and M. S. Brown, in reference to the critical state of research in the U.S. at the start of the 21st century.189) We are currently facing the same situation in Japan.
Without persistent efforts towards clinical applications, discoveries can be wasted at any step in the translational process. If a translational scientist fails in achieving a clinical application for one target, he or she could use that failure to their advantage at the next opportunity, or when examining a different target. Translational science created by experienced translational scientists who are bidirectional and have an excellent eye for judgement in translational research, is unlike basic studies done for no clinical purpose by clinicians or casual remarks on the clinical application of discoveries by basic scientists.
Translational science is a newly emerging science, distinct from basic and clinical sciences in biomedicine. Its field of investigation encompasses the scientific and operational principles underlying each step of the translational process. Advances in translational science increase the efficacy and safety of translational research in all diagnostic, therapeutic, and behavioral applications. This review summarizes the current state of translational research on novel hormones, the natriuretic peptide family and leptin, which has been achieved for clinical application or are in still ongoing in Japan. The importance of translational science is emphasized, as we have learned, from experiences in translational research. In order to advance translational science, it is essential that we train authentic translational scientists, who are bidirectional, “from bench to bedside” or “from discovery to application” and “from bedside to bench” or “from medical practice to laboratory”, and also have an excellent eye for judgement in translational research, with understanding and support from academia, the pharmaceutical industry, and society.
The author is deeply grateful to all of the collaborators in the Department of Medicine and Clinical Science, Medical Innovation Center, EBM Research Center, and Translational Research Center, Kyoto University Graduate School of Medicine, and the Hormone Foundation of Japan, especially Professors Hiroo Imura, Hisayuki Matsuo, Kenji Kangawa, and Shu Narumiya for valuable advice and encouragement. The author is also deeply grateful to patients and their families for their understanding and participation in translational research.
This work was mainly supported by a Grant-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science, and Technology. This work was also supported in part by grants from Smoking Research Foundation and The Hormone Station of Japan. The author declares no other conflicts of interest.
Added in proof: A paper of Phase II clinical trial of CNP analog on patients with achondroplasia presenting its safety and efficacy has been published after the submission of manuscript (N. Engl. J. Med. 2019, 381, 25–35).

Kazuwa Nakao was born in Hyogo Prefecture in 1948 and entered Kyoto University School of Medicine in 1967. During his time at the School of Medicine, he had the chance to be introduced to basic research in biochemistry in the laboratory of late Professor Osamu Hayaishi and learned the pleasure of basic research and gained valuable experience similar to MD-PhD students. He graduated from Kyoto University Graduate School of Medicine in 1973 and entered the Graduate Course of Internal Medicine at Kyoto University headed by Professor Hiroo Imura and did his MD-PhD thesis work on the clinical significance of an opioid peptide, β-endorphin. During this research on opioid peptides, he also received excellent supervision from late Professor Shosaku Numa, an outstanding molecular biologist. These fortune encounters with three excellent mentors in biochemistry, internal medicine, and molecular biology enabled him to become a bidirectional translational physician-scientist, that is, “from bench to bedside” or “from bedside to bench”. He became Professor of Medicine at Kyoto University Graduate School of Medicine in 1992 and retired in 2013. He has performed pioneering translational studies on the natriuretic peptide family (atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide) and on leptin. He has led the development of clinical applications of the natriuretic peptide family and leptin as both diagnostics and therapeutics, and created translational science to promote translational research and trained young translational scientists in Japan. He served as President of the Japan Society of Internal Medicine, Chairman of the Board of Directors of the Japan Endocrine Society, Japan Society of the Study for Obesity, Japan Society of Cardiovascular Endocrinology and Metabolism (CVEM), and Japan Society of Molecular Medicine. He has received Awards from the Japan Endocrine Society, Japan Society of the Study for Obesity, Takamine Award of CVEM, Japan Medical Association Award, Takeda Medical Award and also Medal with Purple Ribbon. He is now Professor Emeritus at Kyoto University, and is working as a researcher in the Medical Innovation Center, Kyoto University Graduate School of Medicine, and the President of certified NPO “The Hormone Station of Japan”. He is an Honorary Citizen of Yabu-City, Hyogo Prefecture.