2023 Volume 99 Issue 1 Pages 1-28
2023 Volume 99 Issue 1 Pages 1-28
Low molecular weight monocarboxylic acids (LMW monoacids, C1–C10) are the most abundant gaseous organic compound class in the atmosphere. Formic or acetic acid is the dominant volatile organic compound (VOC) in Earth’s atmosphere. They can largely contribute to rainwater acidity, especially in the tropical forest, and react with alkaline metals, ammonia, and amines, contributing to new particle formation and secondary organic aerosol production. Gaseous and particulate LMW monoacids were abundantly reported in China. They can be directly emitted from fossil fuel combustion and biomass burring; however, the secondary formation is more important than primary emissions via the photochemical oxidation of anthropogenic and biogenic VOCs. In this paper, we review the distributions of LMW monoacids from urban, mountain, and marine sites as well as from rainwater and alpine snow samples and discuss their sources and formation mechanisms in the atmosphere. We also discuss their importance as cloud condensation nuclei (CCN) and provide future perspectives of LMW monoacids study in the warming world.
In the early 1980s, when this author started his science career as a geochemist at the University of California, Los Angeles (UCLA) as a postdoctoral scholar, acid rain was a major concern of environmental science, especially in Europe and North America. In industrialized countries, people have discovered that forests are damaged, with thousand trees being died. Acid rain and fog have been considered to be harmful on terrestrial plants and aquatic animals, causing the decline of forest and aquatic ecosystems, which include lakes and rivers,1)–3) as well as causing the serious deterioration of the building materials such as marble structures and bronze statues.4) At that time, it was considered that acid rain was mainly caused by sulfuric and nitric acids, which are derived from the burning of fossil fuels.1) Acid rain was recognized as one of the serious social problems although global warming and the subsequent climate crisis have not emerged in public yet.5)
In those days, it was believed that sulfuric acid (H2SO4) was the main cause of rain and fog acidity, which is mainly derived from sulfur dioxide (SO2) emitted from the combustion of fossil fuels such as coal and oil and its subsequent atmospheric oxidation to H2SO4 largely in aqueous phase. Sulfur (S) contents in coals range from 0.5% to 5%, depending on the quality, while contents in crude oils range from 0.5% to 10% (typically 1%–4%).6) Fossil fuel sulfur (S) is present as different chemical forms, including elemental sulfur (S8), thiophene, and other S-containing organic compounds.6) Elemental sulfur (S8) is produced in bottom sediment systems of lakes and ocean where oxygen is consumed through bacterial oxidation of organic matters in settling particles of phytoplankton detritus and surface sediments. In the surface sediments, S-reducing bacteria utilize the oxygen from sulfate (SO42−) to oxidize organic matter, resulting in elemental sulfur via H2S as an intermediate.6)
In a volcanic area, free sulfur (elemental sulfur) is abundantly supplied to the sediments (up to 40% of dry weight sediments), e.g., in acidic Lake Katanuma from Japan.7) Sulfates are abundant in the ecosystems of lakes, ocean, and bottom sediments. During diagenesis and catagenesis in deep sediments as a result of thermal gradient in the oil fields, elemental sulfur reacts with various organic compounds to produce S-containing organic compounds such as thiophenes and thiols during the formation of crude oils and coals.6) In contrast, atmospheric nitric acid is largely produced by photochemical oxidation of nitrogen oxides (NOx), which are emitted from the combustion process of fossil fuels and biomass during high-temperature oxidation of atmospheric N2 (thermal nitrogen) and nitrogen-containing organic matter (fuel nitrogen).8)
Although inorganic acids (i.e., sulfuric acid and nitric acid) are believed to be a major source of acid rain in urban areas and the surroundings, organic acids, including formic and acetic acids, have been abundantly reported in rainwaters from remote sites such as Amazon forest9) and rain and fog samples from Southern California.10) Weak organic acids (formic and acetic acids) could contribute 64% of free acidity and 63% of total acidity of the precipitations during the 1981–1982 wet seasons at Katherine, Australia.9) In the Amazon forest, formic and acetic acids explained more than 90% of rainwater acidity.11) Formic acid can be abundantly produced by ants in a tropical region12); however, its major source is suggested to be photochemical oxidation of various organic compounds (e.g., isoprene) emitted from higher plants. However, in the past, there are few studies of LMW monoacids to understand the geochemistry and atmospheric chemistry of organic acids.
Due to the significant innovation in the internal combustion engine systems of automobiles for last few decades and the recent emission controls of sulfur from power plants where coals are mainly combusted, the problem of acid rain derived from anthropogenic sulfuric and nitric acids has significantly been improved in major cities of the world, including China. For example, at the sampling site of Japan Aerospace Exploration Agency (JAXA) station at Chichijima Island in the western North Pacific, we found an increasing to decreasing trend change of sulfate and nitrate concentrations in marine aerosols over Chichijima Island around 2006.13) The western North Pacific is an outflow region of gaseous and aerosol pollutants from East Asian countries, including China, Korea, and Japan. The sulfate concentrations in winter/spring are enhanced because Asian outflows are enlarged by the intensified westerly wind system in winter to spring. The decline of sulfate and nitrate after 2006 may largely be influenced by the strict control of the Chinese government to decrease the emission of sulfur from power plant facilities and control the emission of NOx in automobile engines.
Although elemental carbon (EC) in Chichijima aerosols showed a decreasing trend from 2001 to 2012 with seasonal peaks in winter/spring, organic carbon (OC) contents in the aerosols did not show a decline, rather indicating the decadal increases in OC/EC and OC/TC (total carbon) ratios.14) Interestingly, oxalic acid and pyruvic acid concentrations were found to increase slightly for the observation period from 2001 to 2013.15) Although LMW monoacids were not measured yet, the observations in the western North Pacific suggest a regime shift from inorganic to organic aerosols that may have occurred in East Asia and possibly on a global scale.
In this paper, we review the studies of low molecular weight monocarboxylic acids (LMW monoacids) in gas and aerosol phases in the atmosphere from different environments as well as from rain, fog, and snow samples to update our knowledge on the sources and formation mechanisms of LMW monoacids and their atmospheric and geochemical importance. This review includes field-based observations from different regions and laboratory and modelling studies. Chebbi and Carlier16) reviewed observational studies on mono- and dicarboxylic acids in the troposphere more than two decades ago. The previous review paper may be helpful to better understand how our understanding on monoacids has advanced for last few decades. More recently, Kawamura and Bikkina17) published a review paper focused on LMW diacids in aerosols, rainwater, snow, and ice core, covering the field campaigns from different regions in the world, which may also be interesting as a companion paper of the current review on LMW monoacids in the atmosphere. The present review on LMW monoacids would help to better understand the atmospheric chemistry of organic aerosols and related gas/particle partitioning of volatile/semi-volatile organic acids in the ambient atmosphere and their roles in atmospheric and climate sciences.
Several techniques were developed to determine volatile/semi-volatile organic acids in rainwater in the early 1980s. Ion chromatography (IC) is the most commonly used method.18) It has an advantage of directly injecting the rain and fog samples without preconcentration. However, in general, the target organic acids are limited to formic and acetic acids due to the conductivity detector used for IC, which is not sensitive enough and often suffers with impurities (other species) in the sample. Because the levels of formic and acetic acids are high enough in rain and fog samples compared with impurities, IC is useful for the measurements of such small monoacids. Capillary gas chromatography (GC) with flame ionization detector (FID) can be used to determine a homologous series of monoacids up to C10 species, in which preconcentration of the samples is required followed by derivatization of carboxylic acids to esters. Although GC/FID is more complicated than IC, its high sensitivity and resolution are excellent for the detection of a homologous series of monoacids (C1–C10) including various isomers.
2.1. GC/FID method for LMW monoacids utilizing p-bromophenacyl esters.Using GC that employs benzyl ester derivatization technique, monoacids (C1–C10) were first reported in rainwater samples from New York, with a predominance of formic and acetic acids.19) Although their concentrations ranged from 1.1 to 16.5 µM, organic acids were concluded to produce a negligible contribution to rainwater acidity. Inorganic acids such as sulfuric and nitric acids are the major contributors. The GC/FID technique had not been used in other rainwater studies. A new analytical technique was developed using a capillary GC and GC/MS to determine C1–C10 monoacids in rain and fog samples from Los Angeles by employing p-bromophenacyl esters of monoacids.10) The same esters were used to detect C1–C5 monoacids in aqueous samples using a packed column GC,20) where the GC resolutions are not good to detect larger organic acids. Because the molecular weights of monoacid p-bromophenacyl esters are much larger than those of benzyl esters, we can minimize the evaporative loss of small esters, such as formic and acetic acids, using p-bromophenacyl esters.
The principle of this unique derivatization technique is characterized by the esterification of RCOOK using α,p-dibromoacetophenone as a derivatizing reagent and dihexyl-18-crown-6 (crown ether) as a catalyst. This crown ether specifically forms a complex with potassium (K) by taking this ion in the crown cage. The crown ether cage traps K+ ion from RCOO−K+ and then reacts with α,p-dibromoacetophenone to form p-bromophenacyl esters at 80 °C in acetonitrile solution (Scheme 1). Rain and fog samples are subjected to a cation exchange resin to prepare the potassium forms of monoacids.10)

Reaction of carboxylate (RCOOK) with α,p-dibromoacetophenone (reagent) and dihexyl-18-crown-6 (catalyst). The latter first forms a complex with K+ ion (potassium-caged crown ether) and then reacts with the reagent to derive p-bromophenacyl ester.10)
Generally, rainwater (25–50 ml) and fog water (1–2 ml) samples were placed in a pear-shaped flask (50 ml) and then adjusted to pH = 8.5–9.0 with 1 M KOH solution. The samples were concentrated into 1 ml using a rotary evaporator at 50 °C under vacuum. The concentrates were transferred onto a Pasteur pipet column packed with cation exchange resin (AG 50W-X4, 100–200 mesh, K+ form). The carboxylic acid-K forms were eluted with ultrapure water into a 50-ml pear-shaped flask and were dried using a rotary evaporator under vacuum and nitrogen blowdown system. Acetonitrile (4 ml) was added into the flask, followed by α,p-dibromoacetophenone (0.2 µmol/µL, 50–200 µl) and a dicyclohexyl-18-crown-6 solution (0.02 µmol/µL, 50–100 µl). It was then heated at 80 °C for 2 h in an ultrasonic bath. The p-bromophenacyl ester derivatives were purified on an SiO2 column, dried under nitrogen gas flow, and then dissolved in n-hexane (50–200 µl). Approximately 2 µl of the hexane solution was injected to a capillary GC. Figure 1 shows a typical capillary GC chromatogram of the p-bromophenacyl esters of authentic C1–C7 monoacids including branched chain (iso) isomers. More detailed procedures can be found in Kawamura and Kaplan21) and Mochizuki et al.22)

Capillary gas chromatogram of LMW monoacid (C1–C7) p-bromophenacyl esters. iC4 means isobutanoic acid or isobutyric acid (2-methylpropanoic acid). The same for iC5 and other branched chain acids.
As summarized in Table 1, LMW monoacids are dominant (ca. 200 µg/L) organic species detected in urban rainwater, where major compounds are categorized from three sources (biogenic, anthropogenic, and photochemical origins). In addition to LMW monoacids, various organic compound classes were detected in Los Angeles rainwater, whose possible sources and approximate concentrations are also presented. Among the biogenic components, carbohydrates (ca. 100 µg/L) are the most abundant class, followed by amino acids (ca. 20 µg/L) and fatty acids (ca. 10 µg/L), which are all derived from plants. Branched chain fatty acids and β-hydroxy fatty acids, which most likely originated from bacteria, are detected as trace components in rainwater with a concentration level of less than 1 µg/L. Biogenic aromatic acids such as p-coumaric and dehydroabietic acids are also present as minor species (<1 µg/L), and they have been used as organic tracers of plant resin and vascular plants (e.g., Refs. 23–25).
| Compounds | Possible sources | Concentrations (µg/L) |
|---|---|---|
| Biogenic | ||
| Carbohydrates | Plants | 100 |
| Free amino acids | Bacteria | 20 |
| Normal fatty acids (>C12) | Plants, bacteria | 10 |
| Branched chain fatty acids | Vascular plants | <1 |
| Dehydroabietic acid | Bacteria | <1 |
| p-Coumaric acid | Vascular plants | 1 |
| β-Hydroxy acids | Bacteria | <1 |
| n-Hydrocarbons (C25–C35) | Plant waxes | 1 |
| Anthropogenic | ||
| LMW aldehydes (C1–C4) | Automobile emission, combustion | 50 |
| LMW monocarboxylic acids (C1–C10) | Automobile emission, combustion | 30 |
| LMW dicarboxylic acids (C2–C10) | Automobile emission, combustion | 20 |
| Unresolved hydrocarbon mixture (UCM) | Fossil fuel combustion, automobile emissions | 20 |
| Phenols | Automobile emissions | 10 |
| Benzoic acid | Automobile emissions | 5 |
| Ketones | Fossil fuel combustion | 3 |
| Phthalates | Plasticizers | 3 |
| Azaarenes | Petroleum, combustions | 2 |
| n-Hydrocarbons (C13–C20) | Petroleum, combustions | 1 |
| Polycyclic aromatic hydrocarbons | Automobile emissions | 1 |
| Polycyclic aromatic ketones | Diesel exhausts | 1 |
| Benzaldehydes | Automobile emission | 1 |
| Halocarbons | Industries | 1 |
| Quinones | Petroleum, automobile emissions | 1 |
| In situ photochemical production in gas and liquid phase | ||
| LMW monocarboxylic acids (C1–C10) | Aldehydes and unsaturated hydrocarbons | 200 |
| LMW aldehydes (C1–C4) | Biogenic and anthropogenic unsaturated hydrocarbons, isoprene | 15 |
| LMW dicarboxylic acids (C2–C10) | Biogenic and anthropogenic unsaturated hydrocarbons, isoprene | 5 |
This table was modified from Kawamura and Kaplan (1991)26) and Kaplan et al. (1985).110)
Carbohydrates and amino acids were measured by HPLC after hydrolysis with 2 M HCl at 100 °C for 5 h.
Aldehydes were determined by HPLC using 2,4-dinitrophenylhydrazine (DNPH) derivatives.111)
Among anthropogenic organics in rainwater, LMW aldehydes (C1–C4, ca. 50 µg/L), monoacids (C1–C10, ca. 30 µg/L), and dicarboxylic acids (C2–C10, ca. 20 µg/L) have been detected as major water-soluble organic compound classes. The lowest or second lowest molecular species is often most abundant in each classes, e.g., formaldehyde (C1) or acetaldehyde (C2) in the aldehyde group, formic (C1) or acetic (C2) acid in the monoacid group, and oxalic acid (C2) in the dicarboxylic acid group. In the organic solvent extractable fraction, the unresolved complex mixture (UCM), which is composed of branched and cyclic hydrocarbon mixtures, is abundantly detected in hydrocarbon fractions. This UCM hydrocarbon appears as a large hump on a capillary gas chromatogram of the hydrocarbon fraction superimposed by a series of normal alkane (C15–C36) peaks.26)
In Los Angeles rainwater, UCM hydrocarbon concentrations (ca. 20 µg/L) are in general one order of magnitude higher than those of n-alkanes. UCM hydrocarbons are abundantly present in biodegraded crude oils6) and enriched in engine oil fraction isolated from crude oils. They are emitted as automobile exhausts to the atmosphere. However, no hump was detected on a gas chromatogram of hydrocarbon fraction in a remote snow sample collected from Mt. Pinos in Southern California mountain region, in which n-alkanes showed a strong odd/even carbon preference index (CPI, concentration ratios of odd-carbon-numbered n-alkanes over even-carbon-numbered n-alkanes) of 5.0. In contrast, a typical Los Angeles urban rain showed a CPI value of 1.4.27) Higher plant waxes show CPI values of 10 or more, whereas petroleum shows values close to 1.0.6),28),29)
Other organic compound classes of anthropogenic origin are abundantly present in rainwaters. Phenols (phenol, methyl phenols, and nitrophenols) and aromatic (benzoic and toluic) acids were detected as relatively abundant species (5–10 µg/L), along with aliphatic (e.g., C6–C8) and phenyl ketones and azaarenes (nitrogen-containing aromatics).30) They are present in fossil fuels (e.g., petroleum) and are also produced during the combustion process of fossil fuels (e.g., automobile emissions).30),31) Phthalates are derived from plasticizers, which can be emitted to the atmosphere via photochemical decay of plastic polymers. Polycyclic aromatic hydrocarbons (PAHs) and lower molecular weight n-alkanes (C13–C20) are also present in rainwater. The former are mainly produced by automobile engine combustion of fossil fuels (gasoline and diesel), while the latter are directly emitted as automobile emissions.
Furthermore, a series of alkyl-furanones (γ-lactones; R = C1–C12), benzofuranone, isobenzofuranone, and their alkyl-substituted forms were detected.30) γ-Lactones are intramolecular esters of γ-hydroxy carboxylic acids, which can be produced in the atmosphere as a series of positional isomers of hydroxy carboxylic acids via photochemical oxidation of monoacids by the attack of OH radicals.32) Under acidic conditions that are provided by acid rain or acidic aerosols, the OH group and carboxyl functional group can react within the molecules of γ-hydroxy carboxylic acids to result in intramolecular esters, such as γ-lactones. γ-Lactones are reported in ambient aerosol samples from Tsukuba, Japan,33) and in rainwaters of Los Angeles.26)
Fossil fuel combustion and biomass burning have been considered as important sources of LMW monoacids, diacids, and aldehydes; however, secondary photochemical productions of LMW polar compounds from various organic precursors are more significant than primary sources (see Table 1). In the case of Los Angeles rainwaters, the photochemical productions of LMW monoacids are considered to be two to three orders of magnitude higher than those of primary emissions, including automobile exhausts. This was based on the comparison of estimated annual wet deposition of LMW monoacids from many rainwater analyses and the estimated emissions from automobile exhausts, which were collected using a dynamometer on highway, freeway, and city driving modes from different passenger cars.34)
Four-year observations of Los Angeles rainwater were conducted at the top of Geology Building on the UCLA campus to measure LMW monoacids.34) Table 2 presents the concentration ranges and average concentrations of LMW monoacids together with aldehydes and dicarboxylic acids for comparison. Four-year studies demonstrate that the two major monoacids are formic (av. 6.5 µM) and acetic (5.6 µM) acids, whereas the dominant aldehyde is formaldehyde (6.9 µM), followed by acetaldehyde (0.65 µM). Figure 2 shows the molecular distributions of monoacids together with aldehydes. Interestingly, monoacid concentrations decrease with an increase of carbon numbers. This trend was found for LMW aldehydes. Generally, concentrations of organic acids and aldehydes decrease with an increase of carbon numbers in the Los Angeles rainwaters.
| Compounds | Concentrations (µM) | Frequencies of occurrence (%) | ||
|---|---|---|---|---|
| Range | Average (X ± Sx) | |||
| Monoacids (56 samples) | ||||
| Formic | C1 | 0.11–51 | 6.5 ± 8.7 | 100 |
| Acetic | C2 | 0.20–29 | 5.6 ± 5.9 | 100 |
| Propionic | C3 | 0.02–2.6 | 0.44 ± 0.47 | 100 |
| Isobutyric | iC4 | <0.01–0.49 | 0.046 ± 0.071 | 95 |
| Butyric | C4 | 0.01–0.67 | 0.10 ± 0.13 | 96 |
| Valerie | C5 | 0.01–0.28 | 0.035 ± 0.046 | 95 |
| Caproic | C6 | 0.01–0.40 | 0.059 ± 0.071 | 96 |
| Heptanoic | C7 | 0.01–0.15 | 0.022 ± 0.026 | 88 |
| Octanoic | C8 | 0.01–0.14 | 0.025 ± 0.032 | 93 |
| Nonanoic | C9 | 0.01–0.13 | 0.027 ± 0.026 | 91 |
| Benzoic | C7 | 0.01–0.31 | 0.051 ± 0.066 | 95 |
| Total | 0.33–79 | 13 ± 15 | ||
| Aldehydes (45 samples) | ||||
| Formaldehyde | C1 | 0.85–45 | 6.9 ± 7.2 | 100 |
| Acetaldehyde | C2 | 0.08–4.8 | 0.65 ± 0.72 | 100 |
| Propionaldehyde | C3 | <0.01–0.9 | 0.10 ± 0.20 | 36 |
| Butylaldehyde | C4 | 0.01–0.5 | 0.08 ± 0.14 | 31 |
| Glyoxal | C2 | 0.01–12 | 1.0 ± 2.1 | 80 |
| Methylglyoxal | C3 | 0.01–11 | 0.8 ± 1.8 | 73 |
| Total | 0.87–28 | 9.2 ± 11 | ||
| Diacids (26 samples) | ||||
| Oxalic | C2 | 0.18–28 | 3.9 ± 7.5 | 100 |
| Malonic | C3 | 0.01–5.5 | 0.74 ± 1.6 | 100 |
| Methylmalonic | C4 | <0.005–0.85 | 0.10 ± 0.26 | 15 |
| Maleic | C4 | <0.005–1.8 | 0.25 ± 0.47 | 96 |
| Succinic | C4 | 0.06–7.3 | 1.0 ± 1.8 | 100 |
| Fumaric | C4 | <0.005–2.1 | 0.23 ± 0.51 | 96 |
| Methylsuccinic | C5 | <0.005–1.8 | 0.28 ± 0.49 | 96 |
| Methylmaleic | C5 | <0.005–0.88 | 0.16 ± 0.23 | 96 |
| Glutaric | C5 | 0.02–2.4 | 0.31 ± 0.59 | 100 |
| Dimethylmaleic | C6 | <0.005–0.28 | 0.035 ± 0.031 | 81 |
| 2-Methylglutaric | C6 | <0.005–0.17 | 0.025 ± 0.035 | 69 |
| 3-Methylglutaric | C6 | <0.005–0.24 | 0.034 ± 0.050 | 73 |
| Adipic | C6 | <0.005–1.2 | 0.12 ± 0.26 | 96 |
| Pimelic | C7 | <0.005–0.24 | 0.022 ± 0.056 | 38 |
| Azelaic | C9 | <0.005–0.19 | 0.041 ± 0.049 | 88 |
| Sebacic | C10 | <0.005–0.010 | 0.003 ± 0.008 | 31 |
| Phthalic | C8 | 0.04–1.9 | 0.31 ± 0.43 | 100 |
| 3- or 4-Methylphthalic | C9 | <0.005–0.35 | 0.08 ± 0.11 | 96 |
| Total | 0.43–51 | 7.5 ± 14 | ||
From Kawamura et al. (2001).34)

Distributions of LMW monoacids (organic acids) and aldehydes in Los Angeles rainwaters collected in 1981–1984.34) iC4, iso C4 (2-methyl propionic acid); Benz, benzoic acid; Gly, glyoxal; mGly, methylglyoxal.
In the atmosphere, smaller molecules may be produced more than larger molecules via photochemical processes. In addition, larger molecules are more often attacked by OH radicals via hydrogen atom abstraction due to the presence of more hydrogen atoms per molecule (e.g., C3 and C4 species have more hydrogen atoms than C2). Formaldehyde concentrations are equivalent to those of formic and acetic acids in the Los Angeles rainwater. Meanwhile, the levels of oxalic acid (the smallest dicarboxylic acid), which is the most abundant diacid, are equivalent with or a little smaller than those of formic and acetic acids and formaldehyde (Table 2). α-Dicarbonyls (glyoxal and methylglyoxal) are important sources of oxalic acid via atmospheric oxidation processes.17)
The pH, conductivity, major inorganic ions, major and trace metals, and total organic carbon (TOC) of the selected rainwater samples (n = 28) were measured.35) It was found that LMW monoacid concentrations were higher when pH values (range 3.54–6.11, av. 4.67 ± 0.54) were lower. The same trend was found for sulfate and nitrate.34) As expected, pH was low when precipitation was weak, showing a positive correlation between pH and precipitation amounts (mm). This correlation means that carboxylic and inorganic acids are scavenged from the atmosphere during an early stage of precipitation event.35) Furthermore, the organic and inorganic acids are important components to lower rainwater pH, that is, they are the important sources of protons (H+) of acid rain. The selected rainwater samples from Los Angeles and the surroundings (Valencia, Duarte, and Corona Del Mar; see Fig. 3 for a map) showed that percentage of hydrogen ions potentially dissociated from mono- and dicarboxylic acids to those from organic acids plus inorganic acids (nitrate and sulfate) ranged from 11% to 44%.36) TOC showed an anticorrelation with precipitation (mm), suggesting that organic materials are scavenged at an early stage of wet precipitation and diluted by subsequent rainfall.35) This study demonstrated that LMW monoacids comprise 0.2%–6.2% (av. 2.1% ± 2.2%) of TOC from the Los Angeles rainwaters.

Sampling sites for rainwaters in Los Angeles and surroundings.35) UCLA is located in West Los Angeles, whereas Downtown Los Angeles is located ca. 30 km east (inland) from UCLA with the center of the freeway system. Closed black circles (●) indicate sampling sites.
High abundances of formic (5–18 µeq L−1) and acetic acids (3–9 µeq L−1) were reported in rainwater samples from China,37) India,38) South Africa,39) and Brazil.40) In a rural site (Lin’an) of China, concentrations of organic acids, including oxalate, were 2.6–114 µeq L−1, and the average contribution of those organic acids to precipitation total acidity was reported as 13.7%.37) The concentrations of those organic acids were higher in the growing season (summer and spring) than those in the nongrowing season (autumn and winter), suggesting that vegetations in the Chinese rural site are important sources of atmospheric formic and acetic acids. Formic and acetic acid concentrations were also found to show a good liner correlation (R2 = 0.86) in the Chinese rural site.37) Forest vegetations may be important sources of these organic acids. Stavrakou et al.41) conducted satellite observations of formic acid on a global scale and suggested that boreal and tropical forests are important sources of formic acid in the troposphere. Based on a model experiment, Paulot et al.42) estimated the global sources of formic and acetic acids, i.e., ∼1200 and ∼1400 Gmol yr−1, respectively; however, these values are highly uncertain.
Considering the high concentrations of LMW monoacids in rainwater and fog samples,43),44) it is reasonable to expect high abundances of those organic acids in the atmosphere either as a gas or aerosol phase. When acid rain emerged as a social problem in the early 1980s, no method was developed to detect a homologous series of LMW monoacids in the atmosphere.45),46) Hence, a sampling technique has been developed to collect monoacids from the atmosphere using neutral quartz filter and alkaline filter (KOH-impregnated quartz filter, 47 mm in diameter, Pallflex).47) Due to the volatile characteristics of LMW monoacids, they are expected to exist largely in a gaseous phase; however, no such observation was reported on volatile organic acids in the early 1980s. A two-stage filter device was designed in a series using a two-step filter cartridge, HEPA filter, airflow meter, and pump to sample the particulate and gaseous organic acids (Fig. 4). The first (neutral) quartz filter collects particulate organic acids that are present as a salt form. The second alkaline (KOH-impregnated) quartz filter collects free organic acids that passed the first neutral quartz filter.47)

Schematic diagram of sampling filter cartridge for collecting particulate organic acids and gaseous organic acids using the first filter (neutral) and the second filter (KOH-impregnated), respectively. Typical airflow rate was 10 L/min. Sampling durations are from a few hours to several days depending on LMW monoacid concentrations.
Quartz filters were used after combustion at 500 °C for 4 h to remove organic contamination, rinsed in 0.18 M KOH solution, and then dried in an oven at 80 °C. To avoid any contamination from the ambient air before and after the sampling, all the KOH-impregnated filters and neutral filters were stored in clean glass bottles with a Teflon-lined screw cap. Before the preparation of KOH solution, KOH pellet surfaces were washed with methanol/dichloromethane mixture to remove any adsorbed organic acids (volatile organic acids are abundant in the ambient air). The blank levels of organic acids of this method can be significantly declined by cleaning the KOH pellets. A typical sampling filter cartridge is composed of neutral and KOH-impregnated quartz filters, which are connected online in the cartridge (Fig. 4). After collecting the filter and returning the sample to the laboratory, aliquot of filters was cut in pieces and extracted with organic free ultrapure water (5 ml × 3) under ultrasonication. The combined extracts were pH-adjusted to 8.5–9.0 using 1 M HCl and 0.2 M KOH solutions and pH meter, and they were then concentrated down to 2 ml using a rotary evaporator under vacuum. The carboxylates were subjected to a cation-exchange column (AG 50W-X4, 100–200 mesh, K+ form) and then to p-bromophenacyl ester derivatization using the method described for rainwater analysis (see Scheme 1).
The trapping efficiencies of volatile monoacids (C1, C3, C5, C7) were evaluated to be 71%–82% (average 78% ± 5%) using KOH-impregnated filter and authentic standards for a sampling operation of 90 min.47) Authentic carboxylic acids were injected in the clean glass tube with clean air and finally collected by the KOH-impregnated quartz filter. Similar trap efficiencies of volatile acids were obtained for longer sampling period (17 h). No significant fractionation was found in the molecular distributions of C1–C7 monoacids during the collection of authentic samples. By applying this sampling technique to the ambient air in Los Angeles at a rooftop of UCLA Geology Building, with a modification by adding the third filter (KOH-impregnated), the overall trapping efficiencies of gaseous organic acids on the second and third KOH-impregnated filters combined are estimated to be 99.9% (June 10–11, 1984) and 99.1% (September 21, 1984), respectively, based on the methods proposed by Smith.48) Trapping efficiencies were estimated as 98% and 91% for the second filter alone.
It was also found that only 17% or less of the total LMW monoacids (C1–C10) are present in aerosol particles (collected by the first quartz filter), and the major portion (>83%) of organic acids is present as volatile gases for summer samples (June 10–11, 1984). Interestingly, the percentages of particulate LMW monoacids increased up to 27% for formic acid in an autumn sample (September 21, 1984).47) This result may suggest that gas/particle partitioning of LMW monoacids could be controlled by meteorological parameters such as temperature and humidity and chemical composition of the atmospheric gas and aerosols by forming salts (carboxylates). For example, when aerosol acidity is higher, volatile organic acids may be more present in the gas phase and vice versa. The acid rain in the early 1980s was serious; thus, aerosols could be more acidic, kicking more organic acids from the aerosol to gaseous phase. In these days, LMW monoacids may more likely exist as aerosol phase to interact with atmospheric water vapors as CCN and more involve in cloud formation and wet precipitation processes as well as water cycles in regional and global scales.
3.2. Case studies in Los Angeles and its vicinities.This methodology has been applied to collect atmospheric monoacids from Southern California, including West Los Angeles, Downtown Los Angeles, Newberry Park, and La Habra, using neutral and KOH-impregnated quartz filters. KOH-impregnated quartz filters were used to collect several samples of gaseous plus particulate organic acids. In general, the homologous series of C1–C10 aliphatic monoacids and benzoic acid were detected in all the samples. Their molecular distributions are characterized by a predominance of acetic and formic acids followed by propionic acid. Higher organic acid concentrations decreased with an increase of carbon numbers, except for C5, which is less abundant than C6 (Fig. 5). Interestingly, Downtown Los Angeles samples showed twice higher concentrations (3.2–5.2 ppbv) than UCLA samples from West Los Angeles (1.1–2.6 ppbv), which is closer to the Pacific Ocean. The samples from two other locations showed lower LMW monoacid concentrations (1.2–2.1 ppbv in La Habra and 0.52–0.79 ppbv in Newberry Park) than those from West Los Angeles.49) Newberry Park is located in Santa Monica Mountains, whereas La Habra is located in rural area outside of Los Angeles. These comparisons suggested that the high LMW monoacid levels are related to anthropogenic activities, such as motor vehicle emissions.
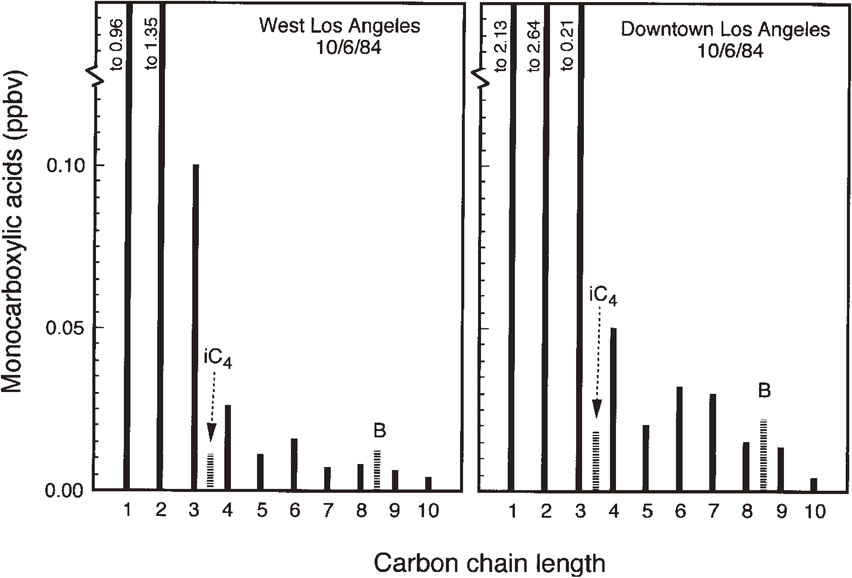
Chain length distributions of LMW monoacids (C1–C10) in the atmosphere of West Los Angeles (UCLA) and Downtown Los Angeles. iC4, isobutyric acid; B, benzoic acid.49) The values mean the concentrations of gaseous plus particulate organic acids.
The LMW monoacids are largely present in gaseous phase, where acetic acid is often more abundant than formic acid. On the other hand, particulate organic acids showed an opposite trend, i.e., formic acid is more abundant than acetic acid in all aerosol samples (n = 14), except for two samples from West and Downtown Los Angeles. This result may suggest that formic acid is preferentially removed from the air via dry deposition as a particle. Gaseous organic acids could form a salt (carboxylate) by reacting with ammonia, amines, or alkaline dust materials. Soil samples (n = 2) were collected from the UCLA campus. However, LMW monoacids, including formic acid, were not detected or were below blank level. In contrast, dust samples collected from Geology Building of UCLA (outside the room window at fifth level) and Downtown Los Angeles showed high abundances of LMW monoacids, with a predominance of formic acid followed by acetic acid. For example, the dust sample from UCLA has the following values: formic acid, 519 nmol/g-dry weight dust; acetic acid, 195 nmol/g-dry weight; propionic acid, 11.5 nmol/g-dry weight; and butanoic acid, 4.4 nmol/g-dry weight.49) These results suggest that gas-to-particle conversion of volatile organic acids, especially formic acid, occurs in the ambient atmosphere, and particles that contain organic acids dominated by formic acid are scavenged from the atmosphere via dry deposition in addition to wet deposition.
Diurnal variations in LMW monoacid concentrations were observed during clear and hot days (max. temperature of up to 40 °C), but such trend was absent during low overcast (cloudy) days. These results suggest that LMW monoacids are largely produced by photochemical oxidations of various organic precursors induced by solar radiation. The secondary production of organic acids is much more important in Los Angeles than primary emissions from automobiles during daytime under strong solar radiation. It is interesting to note that high abundances of LMW monoacids with the predominance of formic or acetic acid were detected in automobile exhausts using a dynamometer and different types of passenger cars as well as the gas/particle sampling technique for organic acids proposed in this paper.49) However, a long-term observation in Los Angeles demonstrated that the secondary formation of LMW monoacids is more important than primary emissions, e.g., automobile exhausts.49)
3.3. Case studies in Sapporo forest.Different stories on gaseous and particulate LMW monoacids in the atmosphere were found based on further studies on gas/particle partitioning during a more recent field campaign in 2010 from Sapporo forest, Northern Japan.50) Simultaneous collection of gaseous and particulate samples was conducted at a deciduous broadleaf forest site in Sapporo to better understand the distributions and sources of LMW monoacids in the forest atmosphere.50) Normal (C1–C10), branched (iC4–iC6), hydroxyl (glycolic and lactic), and aromatic (benzoic) LMW monoacids were detected in gas and particle phases. Figure 6 displays their molecular distributions. Formic (av. 953 ng m−3) and acetic (528 ng m−3) acids were the dominant LMW monoacids in gas phase, followed by propionic (37 ng m−3) and isopentanoic (42 ng m−3) acids. Isopentanoic (iC5, av. 159 ng m−3) acid was dominant in a particle phase, followed by acetic (av. 104 ng m−3), formic (av. 71 ng m−3), and lactic (av. 65 ng m−3) acids. LMW monoacid concentrations did not show correlations with anthropogenic tracers such as nss-SO42− and NO3−, indicating that biogenic sources are more important than anthropogenic sources in the forest site.

Averaged molecular distributions of gaseous and particulate monoacids (C1–C10) in the forest atmosphere from Sapporo, Japan. Samplings were conducted from June 28 to July 8, 2010, with time intervals of 15 h (05:00–20:00LT) in daytime (n = 11) and 9 h (20:00–05:00LT) in nighttime (n = 11). Benz, benzoic acid; iC4, iso C4. Hydroxy acids are shown as glycolic (hC2) and lactic (hC3) acids. Data from Mochizuki et al. (2019).50)
The rate constants of gaseous C1–C4 and iC4 monoacids (provided by NIST Chemical Kinetics Database) were used to calculate the lifetimes of gaseous C1–C4 and iC4 monoacids with OH radicals (OH radical concentration = 2.0 × 106 molecule cm−3).50) The lifetimes of gaseous formic, acetic, propionic, butyric, and isobutyric acids with OH radicals are 12.9, 8.6, 4.8, 3.2, and 2.8 days, respectively. These results showed that organic acids are relatively stable with longer lifetime for shorter-chain monoacids under non-cloudy conditions without precipitation. This unique lifetime feature can in part explain the predominance of formic acid due to the accumulation in gas phase and high concentrations of formic and acetic acids in the atmosphere.
C1–C6 monoacid concentrations in the gas phase showed positive correlations (r2 = 0.21–0.91) with isobutyric acid (iC4) in the forest site.50) Branched chain monoacids, including isobutyric acid, are known as common metabolites of bacteria (e.g., Bacteroides distasonis) and fungi in soils (Ref. 51 and references therein). The forest soil may be a source of gaseous C1–C6 monoacids in the forest atmosphere. LMW monoacids are directly emitted from the combustion of fossil fuel47) and plant leaves52) and are also produced in the atmosphere via photooxidation of anthropogenic and biogenic VOCs.42) In the gas phase, isobutyric acid (iC4) showed positive correlations with C1 (day: r2 = 0.36, night: no correlation), C2 (0.53, 0.43), C3 (0.76, 0.64), C4 (0.82, 0.80), C5 (0.91, 0.81), and C6 (0.72, 0.74) monoacids. C1–C6 monoacid correlations with iC4 suggest that the forest floor is a source of gaseous C1–C6 monoacids in the forest atmosphere. LMW monoacids, such as acetic and propionic acids, can be produced via microbiological processes.51) Exudation of organic acids is known to occur in vascular plants, mainly from roots.53) Shen et al.54) reported that formic, acetic, and propionic acids are present in forest soil and rhizosphere soil.
Although a forest soil sample was not collected from Sapporo during the air sampling campaign, a surrogate soil sample (surface ∼3 cm) was collected from a broad-leaf forest at Chubu University campus in Central Japan on October 31, 2018. The forest floor at Chubu University site is similar to that of Sapporo site in terms of coverage with a broad-leaf litter from similar plant species, including a Japanese oak. The climate in Central Japan is different from Northern Japan, but the air temperature recorded in Chubu University campus in October 2018 (av., 19 °C ± 2.9 °C, Japan Meteorological Agency: https://www.jma.go.jp/jma/indexe.html) was similar to that of Sapporo (21 °C ± 2.3 °C) during the air sampling period. These similarities provide a strong justification to utilize the soil sample from the Chubu University site as a surrogate of the Sapporo forest soil. The surface soil sample was analyzed for LMW monoacids after water extraction employing the analytical protocol described in the experimental section.
Normal (C1–C10), branched (iC4), and hydroxyl LMW monoacids were detected in the soil sample (Kunwar et al., unpublished data, 2018). High abundances of formic (7400 ng gwet soil−1) and acetic (4260 ng gwet soil−1) acids were found, which are significantly higher than the rest of monoacids (∼1800 ng gwet soil−1). Interestingly, hydroxy acids, such as glycolic (1680 ng gwet soil−1) and lactic (1860 ng gwet soil−1) acids, were detected abundantly in the soil sample together with isobutyric acid (77 ng gwet soil−1) (Kunwar et al., unpublished data, 2018). These preliminary results suggest that monoacids in the forest atmosphere are in part derived from forest soil via microbial decomposition of plant debris and subsequent emission to the air. Interestingly, positive correlations between gaseous isobutyric acid and C1–C5 monoacids were found in the Sapporo forest atmosphere in both daytime and nighttime, except for formic acid in nighttime,50) supporting that LMW monoacids are emitted as gases from the soil surface to the forest atmosphere.
To discuss gas/particle partitioning of organic acids, particle phase fractions (Fp) of formic and acetic acids and other species were calculated as Fp = P/(G + P), where P is particle phase concentration and G is gas phase concentration using the Sapporo forest data of G and P concentrations. It was found that Fp of formic and acetic acids negatively correlated with ambient temperature (C1: r2 = 0.49, C2: r2 = 0.60) but positively correlated with relative humidity (C1: r2 = 0.30, C2: r2 = 0.55) in daytime. These results suggest that the meteorological parameters are important for the gas/particle partitioning of monoacids in the forest atmosphere.50) When temperature increases, gas phase concentrations should increase due to the evaporation of volatile organic acids from the particles, whereas particulate concentrations may increase under humid conditions due to the formation of hygroscopic particles. Aerosols enriched with polar organic acids are likely more water soluble and thus are more hygroscopic.
Generally, Fp increased with an increase in carbon chain length of monoacids due to lower vapor pressures of higher MW monoacids. For example, smaller Fp values of formic acid (daytime, 0.08) than acetic acid (0.14) were found, which is consistent with higher vapor pressure of formic acid (5.6 × 10−2 atm) than that of acetic acid (2.1 × 10−2 atm).50) Similar trend and values were reported in the marine atmosphere from the North Pacific.55) In the forest atmosphere, much higher Fp values were observed for nonanoic and decanoic acids (0.49–0.50 in daytime). It is important to note that pH of the water extracts from particle samples was always acidic in the forest samples (pH range, 3.5–6.3; mean, 5.0). Except for propionic acid (r2 = 0.31), the Fp values of LMW monoacids did not show a significant correlation with ambient temperature in nighttime (r2 < 0.16). However, it was found that the average Fp values of LMW monoacids in nighttime were higher than those in daytime (Table 1). Higher temperature promotes the transfer of formic and acetic acids from the aerosol phase to gas phase in daytime via evaporation, which is consistent with their Henry's law constants.
3.4. Case study from the summit of Mt. Tai in the North China Plain.Other stories of LMW monoacids were found via the field campaign at the summit of Mt. Tai (1534 m above sea level) in the North China Plain (NCP) during a large-scale field burning of agricultural wastes (wheat straw) in early summer, i.e., very high concentrations of LMW monoacids and high abundances of particulate organic acids were detected with higher Fp ratio (av. 0.52) for formic acid.22) Gaseous (G) and particulate (P) organic acids were collected in 2006 and analyzed using the method described above to better understand the source and atmospheric behavior of LMW monoacids over Mt. Tai. Normal (C1–C10), branched (iC4–iC6), hydroxy (lactic and glycolic), and aromatic (benzoic) LMW monoacids were detected, in which acetic acid was the most abundant species of the gas phase and formic acid is the dominant species of the particle phase. Formic acid (G, 1570 ng m−3; P, 1410 ng m−3) and acetic acid (G, 3960 ng m−3; P, 1120 ng m−3) concentrations significantly increased during the enhanced field burning of agricultural wastes. Formic and acetic acid concentrations in daytime were found to increase in both G and P phases with those of K+, a good biomass burning tracer (r = 0.32–0.64).
Figure 7 shows the temporal variations in the concentrations of gaseous and particulate organic acids (C1, C2, C3, iC5, and lactic acid) and K+ at the top of Mt. Tai for more field burning (MFB) and less field burning (LFB) periods. The gaseous concentrations of acetic acid during the MFB period (June 2–5) are significantly higher than the LFB period (June 23–26). Similarly, the gas-phase concentrations of formic acid are higher during the MFB period than the LFB period. Moreover, the particle-phase concentrations of formic and acetic acids during the MFB period are slightly higher than the LFB period. It was found that formic and acetic acids in a particle phase were less abundant in the morning and nighttime hours but were maximized around noontime or in the afternoon. This suggests a significant photochemical formation during the upward transport of the field burning plumes from the low land to the top of the mountain. Two peaks of isopentanoic (iC5) and lactic (Lac) acids in a particle phase were observed at 6:00–9:00 on June 3 and 9:00–12:00 on June 4 when the field burning of agricultural wastes increased. However, these acids did not show any diurnal trend in both gas and particle phases.

Temporal variations in the concentrations of major monoacids in gas and particle phases and K+ at the summit of Mt. Tai (height, 1534 m) in the North China Plain.22) Open and solid circles indicate gas-phase and particle-phase samples, respectively. Shaded areas indicate nighttime. The interval of June 2–5 is defined as the more field burning (MFB) period, whereas June 23–26 is defined as the less field burning (LFB) period based on biomass burning tracers (levoglucosan and potassium).
Concentrations of total monoacids in the gas phase ranged from 1530 to 12,100 ng m−3, whereas those in the particle phase ranged from 960 to 9680 ng m−3. Mean concentrations of total monoacids in the gas phase were 6540 ng m−3 and 3070 ng m−3 during the MFB and LFB periods, respectively, whereas those in the particle phase were 4120 ng m−3 and 2850 ng m−3 during the MFB and LFB periods, respectively. These values are several times to one order of magnitude higher than those obtained from the Sapporo forest site. Overall, the gas-phase concentrations of total monoacids are higher than those of particle phase in both MFB and LFB periods. In the gas phase, acetic acid was found as the dominant monoacid species (MFB, 3960 ng m−3; LFB, 1270 ng m−3) followed by formic acid (MFB, 1570 ng m−3; LFB, 890 ng m−3) and lactic acid (MFB, 319 ng m−3; LFB, 433 ng m−3). In the particle phase, formic acid is the dominant species (MFB, 1410 ng m−3; LFB, 883 ng m−3), followed by acetic acid (MFB, 1120 ng m−3; LFB, 763 ng m−3) and lactic acid (MFB, 917 ng m−3; LFB, 661 ng m−3). The mean concentrations of major monoacids in both gas and particle phases are greater during the MFB period than the LFB period, except for gaseous lactic acid and isopentanoic acid (iC5).
These results again demonstrate that field burning of agriculture wastes in the NCP is an important source of LMW monoacids associated with the upward transport and photochemical production during the uplift transport when the planetary boundary layer (PBL) expands in daytime.56) During the campaign periods, concentrations of formic and acetic acids and K+ were higher in daytime than those in nighttime. Because the top of Mt. Tai is below the PBL in daytime, organic acids and other gaseous organic compounds and aerosols are uplifted to Mt. Tai from the lowland of the NCP, where field burning of wheat straws is widely operated in China. The field burning of agriculture wastes shifts from the south to north following the northward shift of harvest season in China, causing a serious atmospheric pollution problem in East Asia.
The correlation coefficients of formic and acetic acids with levoglucosan (anhydrous sugar produced by thermal alteration of cellulose and hemicellulose, a good tracer of biomass burning) were calculated to better understand the effect of biomass burning of agricultural wastes on gaseous and particulate formic and acetic acids.57) Particulate formic and acetic acids in daytime show a weak correlation with levoglucosan during the MFB (C1: r = 0.29, C2: r = 0.28) and LFB (C1: r = 0.28, C2: r = 0.24) periods. This weak correlation may be associated with gas/particle portioning in the atmosphere. However, gaseous formic and acetic acids in daytime did not show a positive correlation with levoglucosan (r = −0.62–0.35). Levoglucosan may be photodissociated in part by OH radicals in daytime.58) In contrast, K+ is emitted from field burning and agricultural activities, which is a more stable open field burning tracer. Particulate formic and acetic acids in daytime showed a positive correlation with K+ during the MFB (C1: r = 0.59, C2: r = 0.64) and LFB (C1: r = 0.44, C2: r = 0.63) periods, whereas particulate formic and acetic acids in nighttime did not show any correlation with K+ (r = −0.29–0.04).
It was found that concentrations of gaseous acetic acid in daytime increased with K+ during the MFB (r = 0.63) and LFB (r = 0.63) periods. Gaseous acetic acid in nighttime showed a positive correlation with K+ (MFB: r = 0.54, LFB: r = 0.39). However, formic acid in both gas and particle phases in nighttime showed no correlation with K+ during the MFB and LFB periods (r = −0.29–0.38). Particulate formic and acetic acids and gaseous acetic acid showed good correlations with K+ but no correlations with levoglucosan. The results suggest that the open field burning in the NCP influences the particulate formic and acetic acids and gaseous acetic acid. This study demonstrated that field burning of agriculture wastes is a significant source of LMW monoacids in the atmosphere of the NCP. Photochemical production of organic acids can also contribute as an important source of LMW monoacids via the oxidation of various organic compounds emitted from the field burning.
Table 3 compares the concentrations of formic and acetic acids in the gas and particle phases from different sites in the world. During the MFB period, the particle-phase concentrations of formic (1410 ng m−3) and acetic (1120 ng m−3) acids over Mt. Tai are significantly higher than those reported in Los Angeles,49) megacities of China such as Beijing59) and Shanghai,60) Amazon forest46) and Taiwan forest,61) and Alaska.62) On the other hand, during the MFB period, the gaseous levels of formic (1570 ng m−3) and acetic (3960 ng m−3) acids over Mt. Tai are lower than those reported in tropical forests from Amazon,46) biomass burning plumes from the Yangtze River Delta region, China,63) urban air from Pasadena,64) and oil and gas fields from Utah.64)
| Locations | Formic acid | Acetic acid | References | |
|---|---|---|---|---|
| Mt. Tai, China (wheat burning) | G | 1570 | 3960 | Mochizuki et al. (2017)22) |
| Los Angeles (urban) | G | 860 | 1800 | Kawamura et al. (2000)49) |
| California, Pasadena (urban) | G | 4000 | — | Yuan et al. (2015)64) |
| Utah (oil and gas producing region) | G | 4700 | — | Yuan et al. (2015)64) |
| Amazon (tropical forest) | G | 3400 | 5900 | Andreae et al. (1988)46) |
| Yangtze River Delta region, China (biomass burning) | G | — | 5000 | Kudo et al. (2014)63) |
| Greenland (mountain) | G | 1070 | 1070 | Dibb and Arsenault (2002)65) |
| France (Alps) | G | 640 | 750 | Preunkert et al. (2007)66) |
| Pacific Ocean | G | 55 | 122 | Miyazaki et al. (2014)55) |
| Antarctica | G | 92 | 75 | Legrand et al. (2012)67) |
| Mt. Tai, China (wheat burning) | P | 1410 | 1120 | Mochizuki et al. (2017)22) |
| Los Angeles (urban) | P | 163 | 120 | Kawamura et al. (2000)49) |
| Amazon (tropical forest) | P | 46 | 48 | Andreae et al. (1988)46) |
| Pacific Ocean | P | 2 | 8 | Miyazaki et al. (2014)55) |
| Taiwan (subtropical forest) | P | 30 | 312 | Tsai and Kuo (2013)61) |
| Alaska | P | 244 | 744 | Li and Winchester (1989)62) |
| Beijing, China (urban) | P | 370 | 350 | Wang et al. (2005)59) |
| Shanghai, China (urban) | P | 80 | 150 | Wang et al. (2006)60) |
G, gas phase; P, particle phase.
The particle-phase concentrations of formic and acetic acids at Mt. Tai are 2–5 times higher than those reported from Los Angeles,49) Greenland,65) and the French Alps66) and 1–2 orders of magnitude higher than those reported from the Pacific Ocean55) and Antarctica.67) These comparisons demonstrate that formic and acetic acid concentrations in particle phases during field burning of wheat straw are extremely high compared with other sites in the world (Table 3). Volatile organic acids may largely be involved with particle formation via the reactions with alkaline components such as ammonia, amines, and basic elements. The later section will further discuss the gas-to-particle formation of volatile organic acids in terms of hygroscopic properties of aerosols and the formation of CCN.
Carbon monoxide (CO) originates from biomass burning68) and is a unique tracer of combustion of fossil fuels and biomass. Average CO concentrations in the MFB and LFB periods were 553 ppb and 467 ppb, respectively.22),69) These levels were very high, which indicates that the air masses over Mt. Tai during the sampling periods were highly polluted. Mean concentrations of total monoacids in both gas and particle phases are greater during the MFB period (P, 4130 ng m−3; G, 6540 ng m−3) than the LFB period (P, 3070 ng m−3; G, 2860 ng m−3). During this campaign, positive correlations were observed between LMW monoacids and CO (r = 0.39–0.64 for formic acid and 0.62–0.70 for acetic acid), demonstrating that field burning of agricultural wastes is an important source of monoacids in the atmosphere over Mt. Tai. A 7-day air mass forward trajectory during the MFB period demonstrated that the dominant air mass flow at a level of 1500 m and 750 m was northeasterly and easterly, indicating outflow of air masses to East Asia and the western North Pacific.22) We suggest that high abundances of monoacids in the particle phases not only degrade the air quality in East Asia but also largely contribute to enhance the hygroscopic properties of ambient aerosols because LMW monoacids are highly water soluble.
Generally, Fp increases with an increase of carbon numbers of monoacids.70) However, the Fp values of formic (0.47–0.52) and acetic (0.24–0.38) acids at the top of Mt. Tai are larger than the values expected from the high vapor pressures of formic (Vp = 5.6 × 10−2 atm) and acetic (Vp = 2.1 × 10−2 atm) acids. In addition, the Fp values of C1 and C2 over Mt. Tai are significantly larger than those reported from the Pacific Ocean (C1, Fp = 0.04; C2, Fp = 0.06)55) and urban Los Angeles (C1, Fp = 0.16; C2, Fp = 0.06),49) in which the same sampling and analytical protocols were used. On the other hand, the Fp value of lactic acid (0.61–0.69) over Mt. Tai is slightly lower than that reported from the Pacific Ocean (0.76–0.82).55)
As a potential reason of higher Fp values for organic acids, the chemical states of those acids in ambient aerosols should be important. For example, ammonia reacts with gaseous acidic species (e.g., oxalic acid).71) Gaseous acetic acid can be adsorbed on calcite (CaCO3), which is a major mineral dust component.72) The vapor pressures of organic salts are significantly lower than those of free organic acids. Therefore, gas-to-particle conversion of organic acids could be an important factor to control their gas/particle partitioning and thus their Fp ratios. However, the Fp of formic and acetic acids did not show any correlation with alkaline species such as Na+, K+, Ca2+, Mg2+, and NH4+ (MFB, r < −0.12; LFB, r < 0.24). The total cation equivalents (Na+, NH4+, K+, Mg2+, and Ca2+) and total anion equivalents (F−, MSA−, Cl−, NO2−, NO3−, Br−, PO43−, and SO42−) were calculated, including normal (C1–C10), branched chain (iC4–iC6), aromatic (benzoic), and hydroxyl (lactic and glycolic) LMW monoacids. However, CO3− and HCO3−, as well as unidentified organic anions, were not considered.
Although correlations were not found between Fp of formic and acetic acids and alkaline species, the excess cations in the aerosols over Mt. Tai could still contribute to the gas-to-particle conversion. Hence, two factors can be considered to enhance the gas-to-particle conversion of formic and acetic acids: one is excess cations, and the other is amines such as dimethylamine derived from fossil fuel combustion and biomass burning.73) Barsanti et al.74) reported that amines can more easily form organic acid salts compared to ammonia. Although amines were not measured in this study, they are derived from vehicle exhaust and animal husbandry (e.g., Ref. 75) and from biomass burning.76) Amines could act as a counterpart for free organic acids, and those amine–organic acid reaction products may contribute to a new particle formation and condensation growth of particles, enhancing the Fp values in the NCP. We suggest that unidentified factors such as amines during atmospheric transport over Mt. Tai may also influence the gas/particle partitioning of formic and acetic acids. A further study is needed to clarify this potential process.
In summary, primary emission and secondary formation of formic and acetic acids are linked with field burning of agricultural residues in the North China Plain. Interestingly, particle-phase fractions (Fp = P/(G + P)) of formic (0.50) and acetic (0.31) acids are significantly high compared with previous studies from Los Angeles and Sapporo, indicating that semi-volatile organic acids largely exist as particles due to the reaction with alkaline components such as ammonia and amines that may also be produced during the process of biomass burning. Field burning of agricultural residues may play an important role in the formation of particulate LMW monoacids in the NCP. High levels of particle-phase lactic acid (917 ng m−3), which is characteristic of microorganism, suggest that microbial activity associated with terrestrial ecosystem may significantly contribute to the formation of organic aerosols over the summit of Mt. Tai during field burning season in the NCP.
As discussed above, LMW monoacids are abundantly present in the atmosphere, with formic acid as the most abundant VOC since it is abundantly emitted from various biogenic and anthropogenic sources. It is also abundant because of atmospheric oxidation of various organic precursors of both biogenic and anthropogenic (including biomass burning) origins, and its lifetime is longer (12.9 days) than acetic (8.6 days) and propionic acids (4.8 days). High abundances of LMW monoacids emitted and produced from various sources can be scavenged from the atmosphere either via dry and/or wet deposition or long-range transport from the source region to remote sites. Snowflakes have more surface areas than raindrops per unit of precipitation weight; therefore, snow can act as a significant sink of LMW organic acids from the atmosphere, especially in the high mountain regions during winter and/or cold regions from high latitudes.
4.1. LMW monoacids in high mountain snow pit samples and long-range transport.To understand the long-range transport of monoacids from the Asian continent to Japanese islands, snowpack samples (n = 10 for 2008, n = 6 for 2009, and n = 11 for 2011) were collected from a pit sequence (depth, ca. 6 m) at the Murodo-Daira snowfield (elevation, 2450 m) near the summit of Mt. Tateyama (elevation, 3015 m), Central Japan, in spring season of 2008, 2009, and 2011.77),78) The high mountains in Central Japan (the Japanese Alps) are located in the outflow region of Asian dusts and pollutants from East Asia (see Fig. 8). The snowpack samples were collected from selected dusty, brown-colored layers and non-dusty white layers in the snow pit sequences and were placed in a clean 8 L glass jar with a Teflon-lined screw cap. Small amounts of HgCl2 were added into the jar to avoid possible microbial activity. The samples were shipped to the laboratory of Hokkaido University (the Institute of Low Temperature Science) and stored in a dark room at 4 °C. In this study, Chinese dust samples collected from Tengger Desert (CJ-1: particle size <250 µm, and CJ-2: <100 µm) in China79) and from Gobi Desert (<10 µm) (Fig. 8) were also analyzed for the comparison of LMW monoacids with snowpack samples.
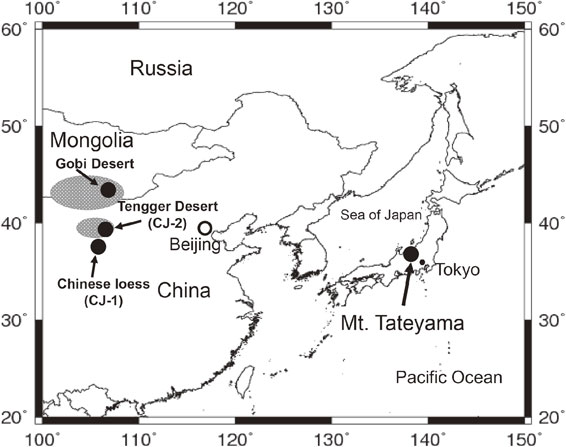
Map of Northeast Asia with sampling site of Mt. Tateyama (elevation, 3015 m), Central Japan, and sampling sites for Chinese dust samples (CJ-1, CJ-2, and Gobi Desert samples) (from Mochizuki et al., 2016).78)
The snowpack samples were analyzed for LMW monoacids with the technique used for wet precipitation samples. A homologous series of normal (C1–C10), branched chain (iC4–iC6), aromatic (benzoic and toluic acid isomers), and hydroxyl (glycolic and lactic) LMW monoacids were detected, with the predominance of formic or acetic acid followed by propionic or isopentanoic acid (iC5). Concentrations of selected organic acids are presented as a function of carbon chain length for typical dust layers and non-dust layers (Fig. 9). Acetic acid is generally more abundant than formic acid. As an important finding, the snowpack samples collected from dusty layers showed higher organic acid concentrations than non-dust layers in the snow pit sequences. This result demonstrates that volatile monoacids are adsorbed by mineral dust particles over China during Asian dust events and cotransported over the Sea of Japan to arrive to the Japanese high mountain regions, where snowflakes scavenge both organic acids and dust particles.

LMW monoacid concentrations in selected snowpack samples (with and without dust layers) collected in 2009 and 2011 from Murodo-Daira site near Mt. Tateyama, Central Japan.78) Snow pit depth was 6 m.
Chinese mineral dusts abundantly contain alkaline metals such as Na, K, and Ca.79) Therefore, volatile organic acids can easily be adsorbed on the dust surface in the atmosphere when a dust event occurs. It is interesting to note that reference dust materials (CJ-1, CJ-2, and Gobi) originally contained formic (up to 4400 ng/g-dust) and acetic acid (up to 18,500 ng/g) abundantly together with nonanoic acid (up to 3200 ng/g).78) The dust particles transported from North China and Mongolia to Japan may contribute to the high levels of organic acids in the snowpack samples with dust layers; however, gas-to-particle conversion of organic acids during the long-range atmospheric transport may be a more significant source in the dusty snow samples. Moreover, it is very likely that volatile organic acids may interact with dust particles to result in the coating of particle surfaces by organic acids when loess deposits are formed in the desert or arid regions of China and Mongolia.
Inorganic ions and dissolved organic carbon (DOC) were also measured for snowpack samples. A strong correlation (r = 0.88) was found between formic plus acetic acids and non-sea salt Ca2+ that is a proxy of Asian dust, as seen in Fig. 10. It was also found that contributions of total monoacids to DOC in 2009 (21.2 ± 11.6%) were higher than those in 2011 (3.75% ± 2.62%) and 2008 (1%–5.9%, av. 3.0%), being consistent with higher intensity of Asian dust in 2009 than in 2008 and 2011. Formic plus acetic acids also showed a positive correlation (r = 0.90) with benzoic acid, which is a tracer of automobile exhaust,47) indicating that monoacids and their precursors are largely emitted from anthropogenic sources in China and/or secondarily produced in the atmosphere by photochemical processing of aromatic hydrocarbons such as toluene.

Scatterplot of formic plus acetic acids and nss-Ca2+ in the snow samples collected from the snow pit sequences (ca. 6 m in depth) near Mt. Tateyama in 2009 and 2011. The dotted line represents the Deming linear regression.78)
The ratios of formic plus acetic acids/nss-Ca2+ for the Murodo-Daira snow pit samples were calculated, and the ratios of formic plus acetic acids/nss-Ca2+ in the reference materials such as CJ-1, CJ-2, and Gobi were compared. Formic plus acetic acids/nss-Ca2+ ratios for the Murodo-Daira snow pit samples from 2009 to 2011 are found to be significantly higher (av. 0.27) than those from CJ-1 (0.00036), CJ-2 (0.0012), and Gobi (0.0018) reference samples collected from the arid areas of North China. These results again indicate that alkaline dust particles can adsorb gaseous monoacids in the atmosphere and that those reactions largely occurred between LMW monoacids and dusts during the long-range transport from the Asian continent to the western North Pacific Rim. Considering a good correlation between monoacids and nss-Ca2+, organic acids in aerosols are very likely to exist in the form of salts, such as Ca(HCOO)2, Ca(HCOO)(CH3COO), and/or Ca(CH3COO)2.
Prince et al.80) reported that gas-phase acetic acid is adsorbed on the surface of CaCO3, a major mineral of dust particles. Acetic acid can form calcium acetate in the atmosphere.72) Vapor pressures of those organic anions are significantly lower than those of free monoacids. In addition, the lifetimes of formic and acetic acids with OH radical are estimated to be 25 days and 10 days, respectively, at −13 °C, assuming the OH concentration of 1.0 × 106 molecules cm−3.42) This timescale is much longer than that of atmospheric transport time of air mass from the Asian continent to Mt. Tateyama. Therefore, the acidity/alkalinity of aerosol surface is an important factor in controlling the uptake of gaseous organic acids. Thus, organic acid salts can be long-range transported as particles in the atmosphere from the Asian continent to the Japanese islands. Although there is a possible volatilization of LMW monoacids during the preparation process of reference materials (e.g., CJ-1 and CJ-2), the vaporization is considered unlikely due to the alkaline nature of dusts. Rather, a potential adsorption of ambient organic acids on the reference materials could not be excluded during the preparation process.
Benzoic acid is directly emitted from fossil fuel combustion81) and also produced in the atmosphere by photooxidation of aromatic hydrocarbons such as toluene,82) which are derived from human activities. Benzoic acid positively correlated with nss-Ca2+ (r = 0.90).83) In addition, the average benzoic acid/nss-Ca2+ ratio obtained for the Murodo-Daira snow pit samples (0.0029) is three to four orders of magnitude higher than those obtained from the Kosa reference materials such as CJ-1 (0.0000024), CJ-2 (0.0000033), and Gobi (0.0000078). Benzoic acid may also be adsorbed on the preexisting particles via atmospheric titration of alkaline dust particles derived from the Asian continent. The air mass trajectories that arrive at the Murodo-Daira site have passed over North China, where many industrial regions and megacities (e.g., Beijing) are located.
Formic plus acetic acids showed a strong positive correlation with benzoic acid (r = 0.90),78) indicating that they are derived from anthropogenic sources in the Asian continent. In contrast, nss-K+, i.e., a tracer of biomass burning,83) did not show a strong positive correlation with formic plus acetic acids (r = 0.18). Even though K+ cannot be formed in the atmosphere, LMW monoacids may be produced largely via secondary photochemical oxidations of various VOCs during long-range atmospheric transport. nss-F−, a tracer of coal burning,59) shows a positive correlation with formic plus acetic acids (r = 0.72), but they were rather scattered.78) These results suggest that burning of biomass and coal is not a major source of monoacids in the snow pit samples collected from the Murodo-Daira site near Mt. Tateyama. Both formic and acetic acids were considered to derive from anthropogenic and photochemical processes in the atmosphere of North China. They are adsorbed on the preexisting alkaline Kosa particles via atmospheric titration during a long-range atmospheric transport over the Japanese Alps and the western North Pacific.78)
The mean concentrations of formic and acetic acids in our samples in 2009 are higher than those reported in mountain snow samples from southern California,35) Tateyama in 2008,77) and south French Alps84) and ice core samples from Antarctica.85) Total monoacid–carbon/DOC ratio (av. 21%) in the Murodo snow pit samples of 2009 is significantly higher than those reported in rainwater samples from Los Angeles (4.4%),34) Shenzhen, China (2.3%),86) and reference dust materials (CJ-1, 2.0%; CJ-2, 2.9%; and Gobi, 3.3%). These results demonstrate that water-soluble LMW monoacids in the snow pit samples near Mt. Tateyama constitute a significant fraction of water-soluble OC, suggesting that entrainment of organic acids in alkaline dusts and snowflakes is significant during the long-range atmospheric transport of dusts from China to Japan. It is likely that the dusts which originated from the desert areas of Mongolia and North China, where reference dust materials (e.g., CJ-1 and CJ-2) were collected, are sources of dust layers in snowpack samples over the Japanese high mountain region.
In summary, the enhanced concentrations of monoacids and nss-Ca2+ were obtained in the snow pit samples with dust layers. It was found that abundances of formic and acetic acids largely depend on non-sea salt Ca2+ (r = 0.88). These acids positively correlated with benzoic acid (r = 0.90), which is primarily produced by fossil fuel combustion and secondary photochemical oxidation of anthropogenic toluene and other aromatic hydrocarbons. This indicates that monoacids in the snow pit samples were mainly of anthropogenic and photochemical origin. Formic plus acetic acids exponentially correlated with pH (r = 0.87) (pH = 4.7–6.9). Alkaline dust particles may be subjected to atmospheric titration by gaseous monoacids. This study demonstrates that Asian dust is a key factor to promote the long-range atmospheric transport of LMW monoacids emitted and produced over North China to the western North Pacific Rim under a strong influence of the East Asian winter monsoon. LMW monoacids can be stabilized against the photochemical decomposition by forming organic acid salts during long-range atmospheric transport.
4.2. Long-range transport of continental air masses from the Indo-Gangetic Plain and Southeast Asia over the Bay of Bengal.To better understand the long-range atmospheric transport of LMW monoacids from the continents to remote oceans, we studied homologous series of LMW monoacids in the fine-mode (PM2.5) marine aerosols (n = 31) collected during a winter cruise (December 2008 to January 2009) over the Bay of Bengal (see Fig. 11), including normal (C1–C10), branched chain (iC4–iC6), aromatic (benzoic acid), and hydroxy acids (lactic and glycolic acids).87) During the winter monsoon, the Northern Indian Ocean receives continental air masses from North India and Southeast Asia via a long-range atmospheric transport. Based on air mass trajectory analyses during the sampling periods, it was found that the aerosol samples were associated with two distinct continental air masses that arrive from the Indo-Gangetic Plain in North India (IGP-outflow) and Southeast Asia (SEA-outflow), as seen in Fig. 11.

(Color online) Cruise track over the Bay of Bengal in December 2008 to January 2009 together with 7-day isentropic air mass back trajectories computed using the HYPLIT model. Blue-colored and black-colored trajectories represent the Indo-Gangetic Plain air masses (IGP-outflow) and the Southeast Asian air masses (SEA-outflow), respectively. Red dots represent the fire spots inferred from the MODIS satellite.87)
The total LMW monoacid concentrations were 59.6 ± 36.4 ng m−3 and 43.1 ± 24.0 ng m−3 in the IGP outflow aerosols and the SEA-influenced aerosols, respectively.87) These concentration levels are significantly lower than those of continental aerosols (e.g., 1430 ng m−3 over Mt. Tai in the North China Plain during field burning season). However, unique molecular distributions of organic acids were found in the marine aerosols over the Bay of Bengal, i.e., they are characterized by the predominance of formic acid (C1) followed by acetic acid (C2) and nonanoic acid (C9) in the IGP-outflow, and a predominance of C9 followed by C2 and C1 was found in some SEA-outflow samples (e.g., January 22 and 23, 2009, see Fig. 12). The predominance of C9 can be explained by the atmospheric oxidation of unsaturated fatty acids such as oleic acid emitted from the ocean surface.88) The molecular distributions of LMW monoacids, which are transported in a long distance from the Asian continent, are different from those of previous studies in Los Angeles, Sapporo, and Mt. Tai in North China as discussed above.

Temporal variations of relative abundances of individual LMW monoacids in the PM2.5 over the Bay of Bengal.87)
The molecular distribution patterns of LMW monoacids are found to be different between two continental air masses from the IGP-outflow and SEA-outflow. Formic-to-acetic acid (C1/C2) ratios were found to be higher than unity (mean: 1.3 ± 0.3) in the IGP-outflow and lower than unity (0.9 ± 0.5) in the SEA-outflow (Fig. 12). These results suggest that the sources of LMW monoacids are different between two continental outflows, i.e., the secondary formation of organic acids is largely important in the IGP-outflow, where anthropogenic activity is very intensive. In contrast, the primary emission is a major source of organic acids in the SEA-outflow, which includes emissions from biomass burning/biofuel combustions, and secondary formations via the oxidation of biogenic VOCs from terrestrial plants or microorganisms. The contributions of biomass burning are supported by the high density of fire spots obtained from the MODIS satellite (Fig. 12) and by a good correlation (r = 0.67) between the concentrations of total monoacids and nss-K+ (biomass burning tracer) in the SEA-outflow.
Based on the correlation coefficient matrix analysis and C1/C2 ratio, we consider that the sources of C1 are probably associated with the secondary formation via the oxidation of biogenic VOCs, while C2 has both primary emission and secondary photochemical formation pathways associated with anthropogenic sources, including biomass burning in the IGP-outflow. On the other hand, as inferred from the MODIS fire spot data (Fig. 12), C1 and C2 in the SEA-outflow have similar sources (both primary and secondary) that originated from biomass burning and bacterial activities via long-range atmospheric transport. These points are supported by high concentrations of isovaleric acid (iC5) of microbial origin and by a significant correlation (r = 0.67) between nss-K+ and total LMW monoacids. The detailed molecular compositions of remote marine aerosols from the Northern Indian Ocean can provide the source information of LMW monoacids produced in the continental atmosphere.
Since LMW monoacids are highly water soluble, they can interact with atmospheric water vapors and thus act as cloud condensation nuclei (CCN), contributing to the formation of fog and clouds44) and affecting radiative forcing of the Earth by reflecting solar radiation via cloud formation.89)–92) They can also contribute to the formation of fog and rain droplet, thus controlling the wet precipitations. Nowadays, water-soluble species (both organics and inorganics) have been recognized as important atmospheric components that act as CCN and contribute to the formation of cloud and fog and initiation of rainfall, controlling water cycles on regional and global scales.93) In case of monoacids and diacids, they can form salts such as ammonium acetate and formate, potassium acetate, or alkylaminium carboxylates in the atmosphere and provide high hygroscopic properties of particles and thus act as CCN.94)–99)
Yu96) reported that although most (98%–99%) volatile organic acids, such as formic and acetic acids, are present in the gas phase, their abundances in the aerosol particles are sufficient to make them good candidates for CCN. More recently, Mochizuki et al.22) discovered the high abundances of LMW monoacids in the particulate phase from the Mt. Tai campaign. The advances of these studies encourage further studies on the size distributions of LMW monoacids in ambient aerosol particles in relation to the CCN properties and the potential contribution of LMW monoacids to CCN and liquid water content (LWC) in aerosols. Although there is no study of CCN and LWC in relation to LMW monoacids in water-soluble organic aerosols, there are several such studies on LMW diacids.99)–103) Hygroscopic properties of organic aerosols and CCN activity were studied in the Asian outflow region104) and the remote marine atmosphere,105) except LMW monoacids.
Organic acids and their salts may provide an important contribution to the formation of CCN from some specific sources, such as vegetation emissions and biomass burning (i.e., forest fires and agricultural waste field burning). LMW carboxylic acids can contribute significantly to the indirect (cloud-mediated) forcing by forming atmospheric aerosols via the reactions with alkaline components and by acting as CCN. Further laboratory studies of hygroscopic properties of organic acids and their various salt forms as a function of particle sizes are needed to better understand the important climatic effects of LMW organic acids. The snow pit samples from dusty layers of Mt. Tateyama suggest that the particle surfaces are often coated with LMW carboxylic acids, thus becoming more hygroscopic. It is very likely that interactions of organic acids and alkaline components in the atmosphere during long-range transport make the particles more hygroscopic and thus more active as CCN.
However, hygroscopic properties of aerosol particles as a function of organic chemical composition, especially at the levels of individual LMW monoacids and their salt forms in different sizes, have not been examined. Being similar to the distributions of LMW diacids in size-segregated aerosol samples,106)–108) it is very likely to presume the size distributions of LMW monoacids in aerosols, e.g., the generally higher abundances in the fine mode and the high abundances in the coarse mode when dusts influence aerosols. To better understand the roles of LMW monoacids in the radiating forcing of the Earth, global water cycles, and climate changes, it is highly recommended to measure LMW monoacids in size-segregated aerosol samples from the urban, forest, mountain, and marine atmosphere, together with the data processing of the measured CCN activity and estimated aerosol water content.
(1) LMW monoacids (C1–C10), which are generally characterized by a predominance of formic or acetic acid, have been found as the most abundant organic compound class in the atmosphere from different sites of the world. They are mostly present in gaseous phase in urban Los Angeles and the Sapporo forest site. Moreover, they are largely found in the particle phase over the summit of Mt. Tai (elevation, 1534 m) in the North China Plain when the field burning of agricultural wastes was conducted after wheat harvesting over the plain. During upward transport from the ground level to the upper troposphere, they can easily react with alkaline species such as ammonia and amines in the air, resulting in the formation of particulate organic acid salts. Volatile organic acids can also be adsorbed on dust particles such as Kosa (Chinese dust) that originated from the arid regions of inland China and Mongolia, abundantly containing alkaline metal ions (e.g., K+, Mg2+, and Ca2+).
(2) The snowpack samples collected from the snow pit sequence (ca. 6 m in depth) in the Murodo-Daira snowfield near Mt. Tateyama (elevation, 3015 m) in the Japanese high mountains (Japanese Alps) demonstrated that high abundances of LMW monoacids were transported from the Asian continent to Japan and scavenged by snow, in which their concentrations were higher in the dusty layers than in non-dusty snow layers in the snow pit sequence. Volatile organic acids react with alkaline components in dust over the continent and transported long distances in the atmosphere as dust particles containing organic acids over the western North Pacific Rim.
(3) These LMW organic acids are efficiently scavenged from the atmosphere via the wet deposition process by rain, fog/mist, and snow. Their concentrations are very high in the wet deposition, which sometimes contribute to 40%–90% of the total acidity, especially in remote areas such as the Amazon forest. Hence, organic acids can largely contribute to lowering the rainwater pH. They are also major source of rainwater OC. For example, LMW organic acids could account for up to 9.5% of dissolved organic carbon (DOC) in Los Angeles rainwaters and up to 35% of DOC in the snow pit sample from Murodo-Daira snowfield. LMW organic acids can also be scavenged via the dry deposition process after the gas-to-particle conversion of organic acids in the atmosphere. Dust deposit samples from Los Angeles and Chinese loess deposits contain high abundances of these acids largely due to the reactions between volatile organic acids and dust particles in the air.
(4) Several sources and pathways produce LMW monoacids in the atmosphere. The combustion process from automobile engines was first proposed as an important emission source of LMW monoacids. However, a comparison study was conducted for the flux calculation of LMW monoacids in Los Angeles basin based on the analyses of large numbers of rainwater samples and estimated emissions of LMW monoacids from the data based on automobile exhaust measurements from different passenger cars using a dynamometer with various driving modes (freeway, highway, and city driving modes). This comparison study demonstrated that direct automobile emissions alone could not explain the large flux of LMW monoacids in rain. This comparison rather suggested that the photochemical productions of LMW monoacids may be two to three orders of magnitude higher than those of primary emissions from automobile exhausts. In addition to anthropogenic hydrocarbons, biogenic VOCs, including isoprene, which is the most abundant biogenic VOC emitted from vegetations, are now recognized as important sources for the production of formic and acetic aids via photochemical processes. Satellite observation also found the high concentration of formic acid over the forest areas, including Amazon, Indonesia, and boreal forests, supporting a large-scale production of volatile organic acids over the forest areas. Microbial oxidation of litters in the surface soil is also suggested as a source of LMW monoacids.
(5) A forest ecosystem is a large factory of global carbon cycles, fixing atmospheric CO2 as terrestrial carbon in trees. At the same time, forest tree leaves emit various VOCs to the atmosphere. Following the recent global warming mainly due to increased CO2 emission by human beings, forest vegetations should be activated to result in more VOC emissions to the atmosphere. The biogenic VOCs are subjected to atmospheric oxidations to result in both gaseous and particulate organic matters. Formic and acetic acids are one of the major reaction products of VOCs. Based on a laboratory ozonolysis of isoprene, a large production of LMW monoacids (C1–C5) was confirmed, with a predominance of formic acid (Kawamura et al., unpublished result, 2016). As a result of global warming, it is likely that atmospheric production of LMW monoacids is increased on a global scale, modifying the physicochemical properties of atmospheric particles. A decadal increase of oxalic acid was discovered based on long-term observation of dicarboxylic acids in the remote marine aerosols from Chichijima Island in the western North Pacific. Oxalic acid is the most abundant diacid and is largely produced by the ozonolysis of isoprene.17),109)
(6) It is likely that the composition of aerosols and gases in the atmosphere may be changing with the regime shift from inorganic to more organic aerosols because of the activated terrestrial ecosystems by global warming and fertilization effect of increased levels of atmospheric CO2 and by the more enhanced pollution controls of SO2 on a global scale causing a decline of sulfate. Although there is no long-term observation of LMW monoacids, it is recommended that Chichijima aerosol samples (1990–2021) should be subjected to extensive analyses of LMW monoacids to test the above hypothesis. Further, more observational, laboratory, and modelling studies are necessary to better understand the roles of LMW monoacids in global carbon cycles as well as the geochemical processes and behaviors of volatile organic acids derived from biogenic VOCs. The roles of water-soluble organic aerosols to act as CCN in the uptake of water vapor to the particles are an important research field in the changing climate system, including global water cycles and the future Earth.
Finally, Fig. 13 shows a graphical illustration on the roles of LMW monoacids as CCN under global climate changes together with the local characteristics for the production pathways or sources of LMW monoacids. Following the recent regime shift from inorganic (e.g., sulfate) to organic (e.g., carboxylic acids) aerosols and more emissions of NH3 from increased usage of fertilizer in agriculture practice, it is very likely that LMW monoacids have more chances to be subjected to gas-to-particle conversion and thus the monoacid-containing particles can largely participate by acting as CCN in changing the processes of cloud formation, wet precipitation/drought, and thus water cycles on a regional and global scales.

(Color online) Graphical illustration on the roles of LMW monoacids as CCN under global climate changes together with local characteristics for the sources and production pathways of LMW monoacids. Biomass burning (forest fires and field burning of agriculture residues) and fossil fuel combustion (automobile and industrial emissions) directly emit LMW monoacids. Biogenic isoprene emitted to the air from terrestrial plants can react with ozone and OH radicals to result in the secondary production of LMW monoacids, such as formic, acetic, and propionic acids. Volatile organic acids can react with alkaline species (e.g., ammonia and amines) to form fine particles, which can act as CCN to form clouds and wet precipitation. During photochemical aging, various gaseous and particulate hydrocarbons and fatty acids are oxidized to result in oxygen-containing organics, including mono- and dicarboxylic acids.
I sincerely acknowledge the late Prof. Ian R. Kaplan (UCLA) and Dr. Robert B. Gagosian (Woods Hole Oceanographic Institution, Massachusetts, U.S.A.) for financial supports and encouragements in science during my earlier studies. I also thank many students, postdocs, and staff for their help and support when I worked at Tokyo Metropolitan University, Hokkaido University, and Chubu University. I thank Dr. Bagawati Kunwar for her preparation of Fig. 13. This study was in part supported by the Japan Society for the Promotion of Science (JSPS) through grant-in-aid Nos. 18K18791, 19204055, and 24221001 and JSPS Joint Research Program implemented in association with DFG (JRPs-LEAD with DFG:JPJSJRP 20181601).
Edited by Eitaro WADA, M.J.A.
Correspondence should be addressed to: K. Kawamura, Chubu Institute for Advanced Studies, Chubu University, 1200 Matsumoto-cho, Kasugai, Aichi 487-8501, Japan (e-mail: kkawamura@isc.chubu.ac.jp).

Kimitaka Kawamura was born in 1951 in Minokamo, Gifu. He graduated from Shizuoka University in 1974 and received a Ph. D. degree from the Department of Chemistry, Tokyo Metropolitan University in 1981 with the title “Geochemical studies of fatty acids and their related compounds in recent sediments”. After taking the postdoctoral position at the Japan Society for the Promotion of Science (JSPS), he moved to the University of California at Los Angeles (UCLA) in 1981 to work with Prof. Ian R. Kaplan as a postdoc and then Woods Hole Oceanographic Institution in Massachusetts in 1985 to work with Dr. Robert B. Gagosian as a visiting investigator. In 1987 he was appointed as an Associate Professor at Tokyo Metropolitan University. He moved to the Institute of Low Temperature Science, Hokkaido University in 1996 as a Professor. In 2016, he moved to Chubu University. He is currently an Emeritus Professor at Hokkaido University and Visiting Professor at Chubu University. He served as a President of Japanese Association of Organic Geochemists from 2011–2015 and a Committee Member and Vice President of the International Committee of Atmospheric Chemistry and Global Pollution (iCACGP) from 1998–2010. He has received several awards/prizes from academic societies including The Japanese Association of Organic Geochemists, Meteorological Society of Japan, Geochemical Society of Japan, Nissan Science Foundation, Geochemistry Research Association (Miyake Award), Elsevier Atmospheric Environment (Haagen-Smit Prize), and Doctor Honoris Causa of Aix-Marseille University. He was elected as a Fellow of the International Union of Pure and Applied Chemistry (2001–2004), Geochemical Society and European Association of Geochemists (2017), American Geophysical Union (2018), and Japan Geoscience Union (2022).