2023 Volume 99 Issue 7 Pages 191-197
2023 Volume 99 Issue 7 Pages 191-197
The mushroom, Pleurocybella porrigens, is widely consumed in Japan; however, in autumn 2004, acute encephalopathy due to ingestion of the mushroom in a large group of patients was reported in Japan. We have continued working on the mushroom to clarify the mechanisms underlying the acute encephalopathy that occurred due to its consumption. The data collected to date have shown that three compounds, pleurocybelline (PC), a Pleurocybella porrigens lectin (PPL), and pleurocybellaziridine (PA), in the mushroom are potentially responsible for the onset of the disease; PC that exhibit lethal activity in mice and PPL formed a complex, and the complex of the two components exhibited proteolytic activity and disrupted the blood-brain barrier. Although PA was not isolated directly from the mushroom, the existence of this compound in the mushroom was predicted. The compound was chemically synthesized and its endogeneity in the mushroom was demonstrated. Furthermore, PA exhibited toxicity to oligodendrocytes.
The mushroom Pleurocybella porrigens (Japanese name, Sugihiratake; English name, Angel-wing mushroom) grows in overlapping clumps on old stumps and fallen trees of conifers, especially cedars, in autumn. It belongs to the family Tricholomataceae and is the only fungus species in the genus Pleurocybella.1) To date, artificial cultivation has not been successful. The mushroom was primarily been eaten in the Hokuriku, Chubu, and Tohoku regions of Japan. However, in autumn 2004, numerous cases of acute encephalopathy due to ingestion of the mushroom were reported. Before this incident, there had been no reports of food poisoning, and the mushroom was widely consumed annually, including by the author, until the incident. In what is considered to be the most severe case of food poisoning in post-World War II Japan, the number of patients reached 59 and 17 people died due to acute encephalopathy. Although the Ministry of Health, Labour and Welfare established a research team to investigate the cause of the disease, none could be elucidated and it was concluded that the cause was “unknown” and the group was dissolved. The author was a member of the research group, and has continued research on the possible causes of the poisoning by ingestion of this mushroom after the dissolution of the group. Recently, we finally elucidated the chemical basis for the mechanism of action and identified three compounds that were involved in onset of the disease.2) Here the author describes the approximately two-decade history of our research on this mushroom.
Immediately after the incident, various possibilities were identified as potential causes of the poisoning. These possibilities ranged from bacterial or viral infection to the mushroom itself, or a mutation in the mushroom. After promptly obtaining a large quantity of the fruiting bodies of this fungus soon after the incident, we examined whether the mushroom itself was toxic to mice by intraperitoneal injection of extracts of the fruiting bodies.
When lyophilized fruiting bodies of P. porrigens were extracted using water at room temperature and then boiling water, respectively, both of the extracts showed lethal activity in mice at 3,000 mg/kg. These extracts were divided into high- and low-molecular-weight fractions by dialysis. Both of the high-molecular-weight fractions showed lethal activity (1,000 mg/kg) in mice, while the low-molecular-weight fractions had no toxicity. The high-molecular-weight fraction extracted with water at room temperature was then boiled for 30 min, and the boiled fraction still showed toxicity. The results indicated that the lethal substance(s) was heat-stable and had a high-molecular-weight. The high-molecular-weight fraction that was extracted with boiling water was then fractionated further using a combination of various chromatography methods guided by the lethal toxicity.
As a result, a lethal compound (lethal activity, 24 mg/kg) showing a single band on SDS-PAGE was isolated.2) The toxin was named pleurocybelline (PC), which is a glycoprotein with a molecular mass of approximately 10 kDa as a monomer. N-terminal amino acid sequencing and liquid chromatograph-tandem mass spectrometer (LC-MS/MS) analyses of PC were unsuccessful, since the glycoprotein was not very soluble in any solvents and buffers.
Arai was first to report the existence of a very specific demyelinating lesion in the myelin sheath of cerebral white matter in the brains of patients who died due to this mushroom-induced encephalopathy.3) Our group and Dr. Arai then initiated a collaborative study and histological examination of the brains of all dead mice did not reveal any abnormalities.
At the time of the incident, lectin (a protein that binds specifically to a sugar or sugar chain and agglutinates red blood cells) activity was much stronger in the mushroom than in other mushrooms, and the “lectin-cause hypothesis” was reported in a major national newspaper, the Mainichi Shimbun, in Japan. Therefore, we purified the lectin (P. porrigens lectin, PPL), determined the primary structure, and cloned the gene.4)–6) This lectin was lethally toxic to rats. However, no brain abnormalities were attributed to PPL in autopsied animals.
The blood-brain barrier (BBB) generally prevents the uncontrolled movement of substances and pathogens into the brain.7) The author considered that the BBB might have been disrupted by the mushroom, causing acute encephalopathy, and we therefore attempted to prove this hypothesis. Thus, PC and PPL were mixed in phosphate buffered saline and examined for protease activity, which was positive. Protease activity was therefore examined using BSA and IgG as substrates by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE). While the BSA band (66 kDa) did not change in response to treatment with PC or PPL (Fig. 1A, lanes 4 and 6), the BSA band disappeared after treatment with PC and PPL in combination (Fig. 1A, lane 7). An experiment using IgG produced a similar result.2) Substrate specificity of the activity of the complex was therefore analyzed using insulin as a substrate by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) analysis. Interestingly, the analysis indicated that this complex showed exo-protease activity with no amino acid specificity and that it degraded the substrate from both N- and C-terminals (Fig. 1B, 1C). This completely non-specific activity was not considered possible in the common sense of enzyme chemistry. Furthermore, Glucose Transporter 1 (GLUT 1) is known to be present in the normal BBB, but GLUT 1 in the BBB disappeared after intraperitoneal injection of the complex, which implies that injection of the mixture into mice damaged the BBB of the animals (Fig. 2).
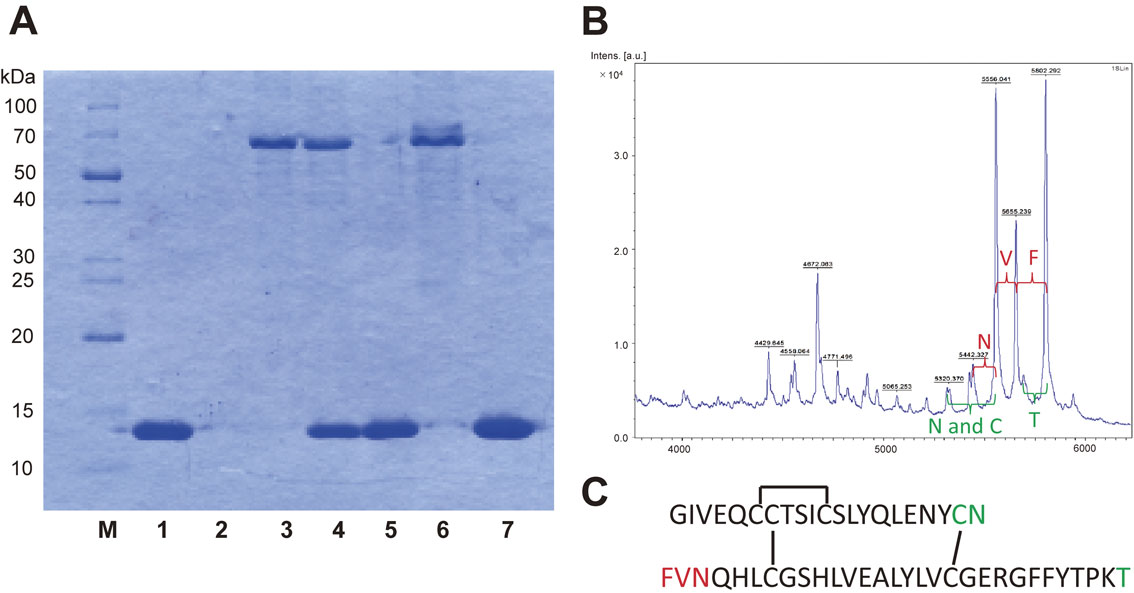
Protease activity of PC and PPL in combination. (A) SDS-PAGE following protease assay using BSA as a substrate. Lane 1: PPL, lane 2: PC, lane 3: BSA, lane 4: PPL and BSA, lane 5: complex (PC and PPL), lane 6: PC and BSA, lane 7: complex (PC and PPL) and BSA, M: molecular mass standards (XL-Ladder Low, APRO Life Science Institute Inc., Tokushima, Japan). (B) MS spectrum of insulin hydrolyzed by PC and PPL in combination. (C) Primary structure of insulin. Hydrolyzed N- and C-terminal amino acids deduced from these molecular masses are shown in red and green, respectively. (Toxicon, 221, 106958, 2023 (Ref. 2))
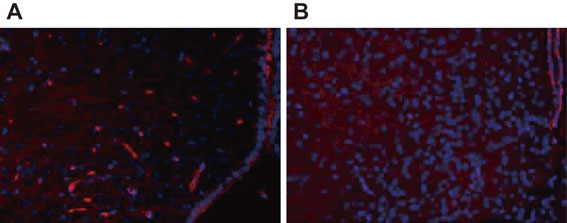
Blood-brain barrier (BBB) disruption by protease activity of the complex. (A) Normal BBB; Glucose Transporter 1 (GLUT 1) was stained red. (B) BBB after intraperitoneal injection of the complex.
There were two clues that led to the author mixing PC and PPL. The first is related to the onset mechanism of influenza-associated encephalopathy. In this infectious illness, trypsin-like proteolytic activity emerges due to the viral infection and disrupts the BBB.8),9) The second reason is the result of a homology search of the amino acid sequence of PPL. PPL is structurally similar to the ricin B chain and HA1 (i.e., the hemagglutinin component of botulinum toxin). Although these two proteins are not toxic themselves, they form complexes with toxins (ricin and the botulinum toxin) and are involved in the development of toxicity.
As mentioned above, a very specific demyelinating lesion in the myelin sheath was observed in the brains of patients who died from encephalopathy. Therefore, the author considered that specific low-molecular-weight compound(s) caused the peculiar demyelinating lesion after passing through the compromised BBB, and we started searching for compound(s) at the same time as the studies on PC and PPL mentioned above. Lyophilized fruiting bodies of the fungus were successively extracted with hexane, EtOAc, EtOH, water, and then boiled water. The EtOH fraction, which was cytotoxic to mouse glial cells, was subjected to chromatography, which resulted in the isolation of three new and unusual amino acid derivatives, as well as three known amino acid derivatives (1–6) (Fig. 3).10) However, these compounds showed only weak cytotoxicity. The author compared the structures of the compounds with one another and postulated the existence of a common precursor (7) (Fig. 3). That is, compounds 1 to 5 are β-hydroxyvaline derivatives, but the positions of the amino and hydroxyl groups are reversed in 6, suggesting that compounds 1–5 were formed by nucleophilic substitution reactions at the β position of 7, while 6 was formed by nucleophilic substitution reactions at the α position of 7. The author then considered this precursor 7 to be the real toxic principle of the poisoning. However, despite repeated analyses of the extracts from the mushroom, we were unable to isolate 7. The author considered that this was due to the instability of its aziridine skeleton, and decided to demonstrate this hypothesis by chemical synthesis.
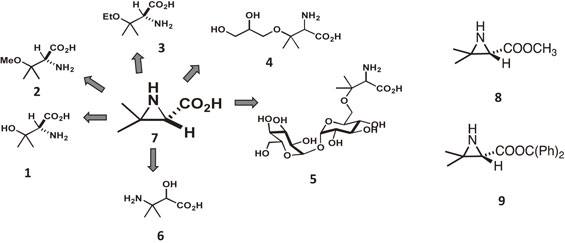
Structures of unusual amino acids isolated from P. porrigens (1–6), pleurocybellaziridine (7), and its esters (8, 9)
The synthesis of 7 was performed by the group of the late Professor Kan.11) First, they synthesized the methyl ester (8) of 7, which we assumed would be more stable than 7 (Fig. 3). Using 8 as an authentic compound, the presence of 7 in the mushroom extracts was demonstrated as follows; fruiting bodies that were collected in 2004, lyophilized and frozen stored were subjected to methanol extraction and then immediately methylated with diazomethane. Thin-layer chromatography analysis of the resulting solution identified a spot that was consistent with the synthetic methyl ester 8. Purification was conducted twice by silica gel column chromatography to obtain a compound whose 1H NMR spectrum was identical to that of the authentic sample, proving that the aziridine-carboxylic acid 7 exists in the mushroom. The methyl ester 8 was not very stable and underwent significant degradation during the concentration and purification steps. In order to demonstrate that aziridine carboxylic acid 7 is the toxic principle that the author expected it to be, 7 itself was synthesized by the Kan group. In this case, diphenylmethyl ester, which can be deprotected under neutral conditions, was selected as the protective group for the carboxylic acid. Due to its steric bulkiness, the diphenylmethyl ester 9 was more stable than 7 and 8 during the concentration step (Fig. 3). Therefore, by comparing the compound obtained by diphenyldiazomethane treatment of the mushroom extracts with synthetic 9, the amino acid was determined to have an S configuration. Deprotection of the diphenylmethyl group in 9 was achieved by a hydrogenation reaction and the desired aziridine carboxylic acid 7 was obtained as white crystals, which was named pleurocybellaziridine. Although the crystals were relatively stable (i.e., more than half a year at −80 °C), significant decomposition was observed in aqueous solution.
As mentioned above, histological observations of the brain tissues affected by the encephalopathy showed demyelination. These findings imply that toxic substance(s) in the mushroom specifically damaged the oligodendrocytes that constitute the myelin sheath in the brain. We therefore examined the toxicity of pleurocybellaziridine (7) and its methyl ester (8) against rat oligodendrocyte cells. As a result, 7 significantly decreased the cell viability while 8 exhibited very weak toxicity (Fig. 4A). Cell staining also indicated that most of the cells treated with 7 showed red fluorescence which represented the dead cells, whereas only a few cells showed fluorescence when treated with 8 (Fig. 4B). Compounds 1 to 6 exhibited markedly less toxicity than 7 (data not shown), suggesting that 7 may be the cause of the demyelinated lesions, and that the carboxylic group and the aziridine skeleton are crucial for the observed cytotoxicity.
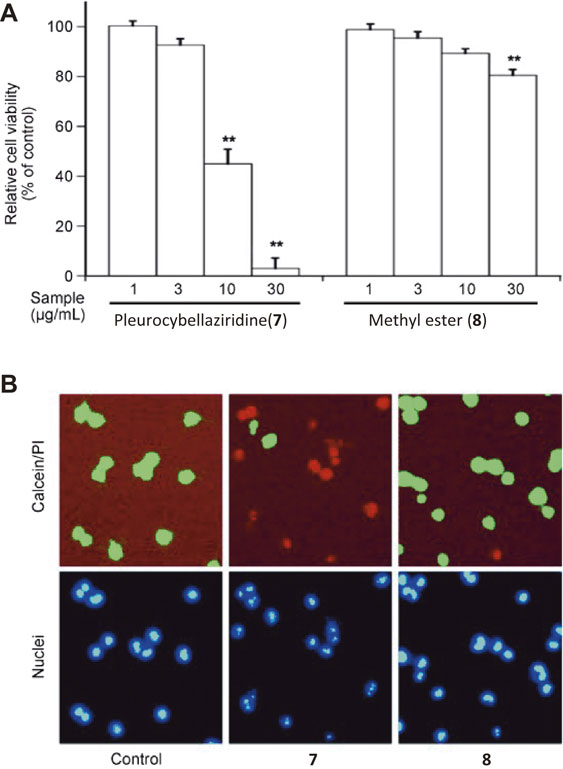
Toxicity of pleurocybellaziridine (7) and its methyl ester (8) to CG4-16 cells. CG4-16 cells treated with 7 or 8 were subjected to an MTT assay (A) and calcein-AM/PI staining (B). (A) The values are represented as the mean of the relative percentage of surviving cells and the standard error of the mean is shown (n = 12; n: the number of each experiment). Statistical analysis was performed by one-way ANOVA followed by a Tukey-Kramer post hoc test. The differences between mean values were considered to be significant when p-values were less than 0.01 (**p < 0.01). CG4-16 cells were treated with 30 µg/mL of 7 or 8 and subjected to live/dead staining. (B) Top panels represent a typical staining pattern of live (calcein, green) and dead (PI, red) cells. Lower panels represent nuclear staining with Hoechst33342 for confirmation that the stained areas in the top panels were actual cells, not artifacts. (Angew. Chem. Int. Ed., 50, 1168–1170, 2011 (Ref. 11))
As mentioned above, the author hypothesized that the complex of PC and PPL destroyed, or functionally disrupted, the BBB. PA then passed through the BBB and showed specific toxicity towards the brain cells, resulting in acute encephalopathy (Fig. 5). The vulnerability of hippocampal neurons to a variety of stresses and pathological conditions has been observed in humans and rodents.12) Hence, the effect of PC, PPL, and PA on mouse brain tissues was examined by intraperitoneal administration of each compound, or various combinations of the compounds, followed by immunostaining analysis.2) The results showed distinct immunostaining signals for the apoptosis marker, single-strand DNA (ssDNA), which was observed in the hippocampus of mice that were treated with the three constituents in combination; however, no distinct immunostaining signals were observed for the other combinations or for each compound in isolation. The number of apoptotic cells detected by anti-ssDNA antibody immunostaining in the hippocampus was significantly increased in the three-compound group compared with those in the control and the other groups (Fig. 6A). Further, immunohistochemical analysis was performed to detect the transfer of His-tagged recombinant PPL (rPPL) into the mouse brain after injection of PC and rPPL. As a result, immunostaining with anti-His tag antibody indicated a very distinct increase in staining intensity in the PC-rPPL combination group compared with those in the PBS (Control) and rPPL groups (Fig. 6B). These results indicate that the administration of the PC-rPPL complex, which possessed protease activity, increased the permeability of the BBB, resulting in the penetration of rPPL into the hippocampus. In addition, the hypothalamus was also stained with the antibody (Fig. 6C) indicating that administration of the complex caused damage both inside and outside the BBB as well. On the other hand, PC, PPL, and PA, either singly or in different combinations, did not change ssDNA-immunopositive numbers in the other brain regions. Immunostaining with anti-NeuN, MAP2, GFAP, and Glut1, which are brain biomarkers for mature neurons, dendrites, astrocytes, and endothelial cells, respectively, showed no significant differences in the brains of mice intraperitoneally administered various combinations of the compounds. All of the results allowed us to conclude that the increase in apoptotic cells in the brain were caused by the three compounds in combination.
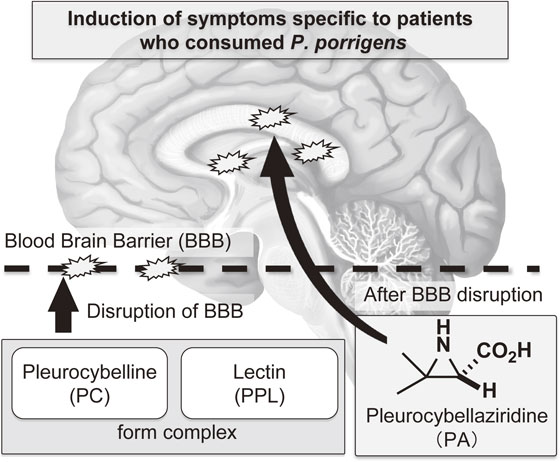
Proposed molecular mechanism of acute encephalopathy following ingestion of P. porrigens. (Toxicon, 221, 106958, 2023 (Ref. 2))

Immunohistochemical analysis of the mouse hippocampus. (A) The number of apoptotic cells in the hippocampus of mice. Control: PBS, PC: PC (24 mg/kg), PPL: PPL (30 mg/kg), PA: PA (70 mg/kg), PC + PPL: PC (24 mg/kg) and PPL (30 mg/kg), PC + PPL + PA: PC (24 mg/kg), PPL (30 mg/kg) and PA (70 mg/kg). Values are mean ± SEM from 3 mice/group. An asterisk (*) indicates significant differences compared to the control (p < 0.05). Detection of His-tagged rPPL in the hippocampus (B) and hypothalamus (C). Control: PBS, rPPL: rPPL (30 mg/kg), PC + rPPL: PC (24 mg/kg) and rPPL (30 mg/kg). (Toxicon, 221, 106958, 2023 (Ref. 2))
We performed whole-genome and transcriptome sequencing of the fungus by next-generation sequencing and constructed the integrated genome database, A-WING.13),14) The results showed that the fungus possesses a unique genome signature and contains numerous novel genes compared with other basidiomycetes.
Even though some chemical studies on the food poisoning caused by the fungus have been reported, the mechanism of acute encephalopathy resulting from the fungus consumption remains unresolved.15)–18) However, from all of the results described in this review, the author considers that the “involvement of three constituents in onset” theory is the most plausible molecular mechanism underlying the cause of the disease caused by angel-wing mushroom ingestion (Fig. 5).
The author would like to thank all of the coworkers involved this study. I am particularly grateful to Dr. Tomohiro Suzuki who received his bachelor’s, master’s, and doctoral degrees under my supervision. The research on the fungus was the main theme of his doctoral thesis and he verified the “three-constituents theory” using animal experiments, which were completed due to his continuous and dedicated efforts. I am also sincerely grateful to the late Professor Toshiyuki Kan (University of Shizuoka) who synthesized PA and its esters. Without his contributions, this study could not have been possible.
This work was partially supported by a Grant-in-Aid for Research and Development Projects for Applications in Promoting a New Policy in Agriculture Forestry and Fisheries from the MAFF, a Grant-in-Aid for Scientific Research on Innovative Areas “Chemical Biology of Natural Products” from MEXT (Grant No. 24102513), and a Grant-in-Aid for Scientific Research (A) from JSPS (Grant No. 24248021).
Edited by Keisuke SUZUKI, M.J.A.
Correspondence should be addressed to: H. Kawagishi, Faculty of Agriculture, Shizuoka University, 836 Ohya, Suruga-ku, Shizuoka 422-8529, Japan (e-mail: kawagishi.hirokazu@shizuoka.ac.jp).
pleurocybellaziridine
PCpleurocybelline
PPLPleurocybella porrigens lectin

Hirokazu Kawagishi was born in 1956 in Kushiro-shi, Hokkaido, Japan. He graduated from the Faculty of Agriculture, Hokkaido University in 1979 and received his Ph.D. from the Hokkaido University in 1985. He began his academic career as Assistant Professor at the Shizuoka University in 1985 and was then promoted to Associate Professor in 1989 and Professor in 1999. He joined the Yoshito Kishi group in 1998 at the Harvard University as Visiting Researcher and worked till 1999. He is now Honorary Distinguished Professor at the Shizuoka University. Publications: Original articles: 277, Reviews: 65, and Book chapters: 28. Prizes: 1) Japan Prize of Agricultural Science from the Association of Japanese Agricultural Scientific Societies (association of fifty academic societies of agricultural science), 2016; 2) The Yomiuri Prize of Agricultural Science from The Yomiuri Shimbun, 2016; 3) The 16th Green and Sustainable Chemistry Award from the Minister of Education, Culture, Sports, Science, and Technology, 2017; 4) JSBBA Award from Japan Society for Bioscience, Biotechnology, and Agrochemistry, 2020; 5) The Medal with Purple Ribbon from the Japanese Government, 2021.