ABSTRACT
Objective: This study aimed to characterize reaching movements of the
paretic arm in different directions within the reachable workspace in post-stroke
patients. Methods: A total of 12 post-stroke patients participated in this
study. Each held a ball with a tracking marker and performed back-and-forth reaching
movements from near the middle of the body to one of two targets in front of them located
on the ipsilateral and contralateral sides of the arm performing the movement. We recorded
and analyzed the trajectories of the tracking marker. The stability of arm movements was
evaluated using areas and minimum Feret diameters to assess the trajectories of both the
paretic and non-paretic arms. The speed of the arm movement was also calculated.
Results: For the paretic arm, contralateral movement was more impaired than
ipsilateral movement, whereas for the non-paretic arm, no difference was observed between
the directions. The maximum speed of the contralateral movement was significantly slower
than that of the ipsilateral movement in both the paretic and non-paretic arms.
Conclusion: The paretic arm shows direction-specific instability in
movement toward the contralateral side of the arm.
INTRODUCTION
Strokes are a major cause of disability worldwide.1) The resulting hemiparesis commonly limits the activities
that can be performed during daily living. Although motor impairments in stroke patients
vary, commonality exists in the aberrant patterns of movement. For example, abnormal synergy
patterns are frequently observed in post-stroke patients, regardless of the size or location
of the brain lesion. The flexor synergy of the upper limb is generally associated with
forearm supination, elbow flexion, and shoulder flexion, whereas the extensor synergy is
responsible for forearm pronation, elbow extension, and shoulder extension.2) Patients’ recovery patterns for
regaining voluntary control of movement can be characterized using the Brunnstrom Recovery
Stage (BRS).3) This framework
suggests that there are common movement patterns in paretic extremities.4) A better understanding of common
movement patterns can assist in the development of an effective rehabilitation program for
hemiparesis.
Deficits in arm movements persist in a large population of stroke patients.5) In particular, abnormal synergies in
the upper limb and the subsequent reduction of available workspace lead to a reduction in
kinetic output.6) Although much
attention has been given to the limited range of motion and limited reachable workspace of
the paretic arm,6,7,8,9) little is known about the characteristics of paretic arm
movement within this workspace. However, considering the stereotypic abnormal synergy
patterns seen in the paretic arm, it is reasonable to assume that the decreased smoothness
and coordination commonly seen in goal-directed movements performed by stroke
patients10,11) exist even within the reachable
workspace. In a previous study conducted by Levin that examined paretic arm movement within
the reachable workspace, interjoint (shoulder–elbow) coordination was demonstrably impaired
in post-stroke patients.12)
However, whether the degree of impairment is influenced by the direction of movement has not
yet been fully elucidated. In this study, by thoroughly investigating paretic and
non-paretic arm movements, we demonstrated that paretic arm movement on a planar surface is
impaired in a direction-specific manner. Specifically, we found that the trajectory of the
paretic arm undergoes greater deflection during target-directed back-and-forth movements in
the contralateral workspace than in the ipsilateral workspace. Consequently, this study
advances our understanding of paretic arm movement within the reachable workspace.
METHODS
Participants
A total of 12 chronic hemiparetic patients who had experienced a stroke incident more
than 6 months earlier participated in this study. Patients were excluded from the study if
they had unilateral spatial neglect, apraxia, shoulder subluxation, or pain. The
characteristics of the participants are further outlined in Table 1.
Table 1.
Clinical and demographic data of the subjects
| Characteristics |
|
| n |
12 |
| Sex (male/female) |
9/3 |
| Age (years) |
65.3±7.4 |
| Dominant hand (left/right) |
0/12 |
| Months since stroke onset |
52.1±50.7 |
| Type of stroke (infarction/hemorrhage) |
6/6 |
| Paretic side (left/right) |
8/4 |
| Brunnstrom Recovery Stage of the arm
(I/II/III/IV/V/VI) |
0/0/2/6/2/2 |
Data are shown as mean ± standard deviation or n.
The arm movement tasks were designed based on the study conducted by Levin, with slight
modifications.12)
Subjects were seated in front of a desk on which three target stickers were attached
(Fig. 1). One target sticker was located near
the middle of the subject’s body, 5 cm away from the proximal edge of desk. The other two
target stickers were placed 25 cm away from the first target: one at 45° and the other at
−45°. The subjects positioned the forearm in the neutral position (pronation at 0°), held
a ball (φ=6.5 cm, 7 g, made of polyvinyl chloride) with a tracking marker, and performed
the following back-and-forth arm movements on the planar surface: (1) diagonal arm
movement, beginning from the proximal target to the target placed on the ipsilateral or
contralateral side of the arm (ipsilateral and contralateral movement tasks), and (2)
horizontal arm movement, beginning from the target on the ipsilateral side to that on the
contralateral side (horizontal movement task). The subjects were asked to perform each
back-and-forth arm movement as fast as possible five times. Patients who could not perform
a seated reaching task without compensatory trunk movement were excluded. In this study, a
compensatory trunk movement was defined as the trunk having crossed over the proximal edge
of the desk during arm movement tasks.

The movement of the tracking marker was recorded at 60 frames/s with a camera (C920r,
Logicool) that was set above the desk. Using motion analysis software (Kinovea 0.8.27,
Kinovea), the trajectories of the tracking marker were depicted and the maximum speeds in
each direction (extension and flexion) were calculated. The area covered by the trajectory
and its minimum Feret diameter (hereafter referred to simply as the Feret diameter) were
calculated using ImageJ (NIH) image analysis software for the ipsilateral and
contralateral movement tasks (Fig. 1B and C).
The area covered by the trajectory and the Feret diameter were analyzed also for the
horizontal movement task. In addition, each trajectory obtained during the horizontal
movement task was divided into two areas by a line extending from the middle target. We
analyzed the trajectory area (surrounded by the trajectory and the line) and the Feret
diameter on each side (ipsilateral and contralateral areas of the horizontal movement
task, Fig. 1D).
Statistical Analysis
The trajectory areas, Feret diameters, and movement speeds for the ipsilateral and
contralateral movement tasks were subjected to two-way repeated measure analysis of
variance (ANOVA) with the arm (non-paretic and paretic) and task (ipsilateral and
contralateral) as within-subject factors. When interaction was detected, Sidak’s multiple
comparison test was performed as post hoc analysis. For the horizontal movement task,
Wilcoxon’s signed-rank test was used to compare the trajectory areas and Feret diameters
of the paretic and non-paretic arms. Furthermore, for the ipsilateral and contralateral
areas of the horizontal movement task (Fig. 1D),
two-way repeated measures ANOVA was used to examine the effect of the arm (non-paretic and
paretic) and side (ipsilateral and contralateral) on the trajectory area and the Feret
diameter. Statistical analysis was performed using GraphPad Prism (version 8). For all
statistical tests, P <0.05 was regarded as statistically significant.
Ethical Considerations
This study was approved by the Research Ethics Committee of SENSTYLE group (#19–001) and
performed according to the principles of the Declaration of Helsinki and its later
amendments. Written informed consent was obtained from the participants before
participation.
RESULTS
The results for the trajectory area and Feret diameter of the ipsilateral and contralateral
movement tasks are presented in Fig. 2, along with
representative images of the trajectories. Two-way ANOVA revealed significant interaction
between arm and task for both the trajectory area (P=0.018) and Feret diameter (P=0.0007).
Post hoc analysis revealed that in the non-paretic arm, no significant difference in the
trajectory area or Feret diameter was observed between the ipsilateral and contralateral
movements (area, P >0.99; Feret diameter, P=0.85). In the paretic arm, however, the
trajectory area and Feret diameter were significantly greater in the contralateral than in
the ipsilateral movements (area, P=0.0045; Feret diameter, P <0.0001; Fig. 2B and C).

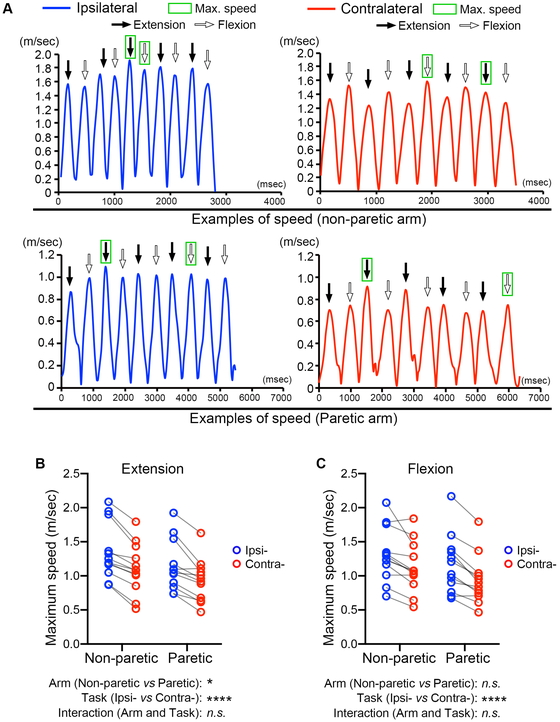
Figure 3A shows representative examples of time
courses of the tracking marker speed for non-paretic and paretic arms during the ipsilateral
and contralateral diagonal movements. The maximum speed of the tracking marker in both
directions (extension and flexion) was identified. For the maximum speed of extension, arm
and task were both revealed to have major effects (arm, P=0.011; task, P <0.0001). For
the maximum speed of flexion, task had a significant effect but not arm (arm, P=0.090; task,
P <0.0001). In both directions, extension and flexion, the interaction between arm and
task was not significant (extension, P=0.62; flexion, P=0.43).
In the horizontal task, the trajectory area and Feret diameter were significantly greater
in the paretic arm than in the non-paretic arm (area, P=0.0010; Feret diameter, P=0.0005;
Fig. 4A and B). Regarding the ipsilateral and
contralateral areas of the horizontal movement task (Fig.
1D), two-way ANOVA revealed that arm had a significant effect of arm (area,
P=0.0011; Feret diameter, P=0.0021) but not side (area, P=0.057; Feret diameter, P=0.67). No
significant interaction between arm and side was found (area, P=0.084; Feret diameter,
P=0.36).
DISCUSSION
In this study, we aimed to identify common movement patterns in post-stroke patients and
showed direction-specific movement disruption in the paretic arm within the reachable
workspace of stroke patients. Specifically, reaching movement toward the contralateral side
of the arm was significantly disrupted compared with movement toward the ipsilateral side in
the paretic (but not in the non-paretic) arm. This investigation not only generated data on
how far the paretic arm is able to reach, but our findings also indicated that the quality
of movement in different directions must be considered when evaluating the paretic arm
during rehabilitation.
Direction-specific movement disruption in stroke patients was previously speculated upon in
a study by Levin in which extension reaching movements (not back and forth) were performed
by ten hemiparetic subjects.12)
In that study, Levin found a significant difference in the movement of the paretic and
non-paretic arms. However, no significant differences were reported between ipsilateral and
contralateral arm movements, although the average values did appear to be different (Fig. 4 in Levin’s study). Consequently, Levin
attributed the apparent difference to the large variability between subjects. In the current
study, we changed the movement task to a series of fast back-and-forth movements and
detected deflection that may have been caused by instability in arm movement. The difference
in our findings compared with those of Levin may be the result of the decreased variety of
movement strategies used in our movement trials.

One possible cause of direction-specific disruption in the paretic arm is the demand of a
particular combination of joint movement patterns. Briefly, in contralateral movement, a
combination of shoulder horizontal flexion and elbow extension is required, whereas in
ipsilateral movement, a combination of shoulder lateral rotation and elbow extension is
required. Difficulty in movement, therefore, might be affected by these combinations. In
concordance with our results, Levin demonstrated that the elbow joint angle in the paretic
arm is disrupted in contralateral movement but not in ipsilateral movement.12) Further work is required to
elucidate the conditions under which paretic arm movements are disrupted. Nonetheless, our
research will serve as a basis for future studies on the common characteristics of paretic
arm movement.
Whereas trajectory disruption in the paretic arm was greater for contralateral movements,
the maximum arm speed was greater in the ipsilateral than in the contralateral movement
tasks for both arms. This finding indicates that motor functions (trajectory and speed in
the current study) are not always equally impaired in the paretic arm. Speed differences
between the directions were also observed in our pilot study with young healthy subjects
(data not shown). Another study with healthy subjects has also demonstrated a lower peak
velocity in pointing movement toward contralateral targets.13) Consequently, it can be speculated that
contralateral movement is kinematically more difficult than ipsilateral movement. Because
the speed and accuracy of movement have a trade-off relationship,14,15,16) slower movement speeds could increase the stability of the
trajectory. In the paretic arm, however, the movement trajectory was disrupted at a slower
speed in the contralateral than in the ipsilateral movement task, further supporting the
hypothesis that there is direction-specific movement disruption in the paretic arm.
Because the contralateral target is far from the shoulder joint of the task-performing arm,
the accuracy of arm movement may depend on the distance from the shoulder joint. To clarify
this issue, we divided the trajectory area obtained during the horizontal movement task into
two areas by a line extending from the middle target, and compared the horizontal arm
movements between these two areas. As a result, we found no difference in the trajectory
area or Feret diameter between the workspaces on the ipsilateral side (closer to the
shoulder joint) and on the contralateral side (further from the shoulder joint). Therefore,
it appears that paretic arm movement is disrupted in a movement direction-specific manner
and not in a workspace-specific manner. However, in this study, the horizontal trajectories
were analyzed only by splitting the series of movements. More sophisticated investigations
may be necessary.
Overall, our data imply that there is direction-specific disruption in the paretic arm
within the reachable workspace. However, some limitations of the current study should be
noted. First, our sample size was limited to 12 participants, and a larger sample may have
strengthened our results. Second, the precise lesion sites were unknown because of the time
that had elapsed from stroke onset and the limitation and regulation of our facility
providing only self-pay rehabilitation services (i.e., not covered by health insurance).
Consequently, there was no direct access to patient medical information. Although our
results identified characteristics of paretic arms with statistical significance, we cannot
completely deny the possibility that differences in the lesion site could have influenced
our results. Finally, this was not a longitudinal study, and therefore, it is unknown
whether the disruption we revealed can be ameliorated during the recovery process. In
contrast to Levin’s study,12)
we were able to detect direction-specific movement disruption in the paretic arm. Even
subjects who were ranked as BRS VI demonstrated significant disruption in movement toward
the contralateral side when using the paretic arm. It is not surprising, however, that
direction-specific movement disruption in the paretic arm persists over a long period.
Because the contralateral workspace for the paretic arm is ipsilateral workspace for the
non-paretic arm, the paretic arm is used less for reaching toward the contralateral side.
This could be the cause of learned non-use of the paretic arm that often develops in chronic
stroke patients.17,18,19) A rehabilitation program that involves handling
objects in, or reaching toward, the contralateral workspace might alleviate the progress of
direction-specific impairment.
CONCLUSION
The present study indicates that the paretic arm in post-stroke patients has
direction-specific instability during movement to the contralateral side of the arm. The
quality of movement within the reachable workspace may be improved not only by extending the
reaching distance but also by improving the movement trajectory.
ACKNOWLEDGMENTS
This work was supported in part by the Higo Foundation for Promotion of Medical Education
and Research. The authors thank Sae Nishimura, Kana Nitta, and Takato Hidaka (Kumamoto
Center, Rehabilitation Center for all Customers with Stroke and Cerebrovascular Diseases)
for their help in conducting this study.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
REFERENCE
- 1. Di Carlo A: Human and economic burden of stroke.
Age Ageing 2008;38:4–5. PMID:19141505, DOI:10.1093/ageing/afn282
- 2. Twitchell T: The restoration of motor function
following hemiplegia in man. Brain 1951;74:443–480. PMID:14895765,
DOI:10.1093/brain/74.4.443
- 3. Brunnstrom S: Motor testing procedures in
hemiplegia: based on sequential recovery stages. Phys Ther 1966;46:357–375. PMID:5907254,
DOI:10.1093/ptj/46.4.357
- 4. Roh J, Rymer WZ, Perreault EJ, Yoo SB, Beer RF:
Alterations in upper limb muscle synergy structure in chronic stroke survivors. J
Neurophysiol 2013;109:768–781. PMID:23155178, DOI:10.1152/jn.00670.2012
- 5. Hendricks HT, van Limbeek J, Geurts AC, Zwarts
MJ: Motor recovery after stroke: A systematic review of the literature. Arch Phys Med
Rehabil 2002;83:1629–1637. PMID:12422337, DOI:10.1053/apmr.2002.35473
- 6. Beer RF, Ellis MD, Holubar BG, Dewald JP: Impact
of gravity loading on post-stroke reaching and its relationship to weakness. Muscle Nerve
2007;36:242–250. PMID:17486581, DOI:10.1002/mus.20817
- 7. Kurillo G, Chen A, Bajcsy R, Han JJ: Evaluation
of upper extremity reachable workspace using Kinect camera. Technol Health Care
2013;21:641–656. PMID:24284552, DOI:10.3233/THC-130764
- 8. Kurillo G, Han JJ, Abresch RT, Nicorici A, Yan P,
Bajcsy R: Development and application of stereo camera-based upper extremity workspace
evaluation in patients with neuromuscular diseases. PLoS One 2012;7:e45341. PMID:23028947,
DOI:10.1371/journal.pone.0045341
- 9. Andrews AW, Bohannon RW: Decreased shoulder range
of motion on paretic side after stroke. Phys Ther 1989;69:768–772. PMID:2772040,
DOI:10.1093/ptj/69.9.768
- 10. Levin MF, Liebermann DG, Parmet Y, Berman S:
Compensatory Versus Noncompensatory Shoulder Movements Used for Reaching in Stroke.
Neurorehabil Neural Repair 2016;30:635–646. PMID:26510934,
DOI:10.1177/1545968315613863
- 11. Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B,
Frontera WR, Stein J, Hogan N: Movement smoothness changes during stroke recovery. J
Neurosci 2002;22:8297–8304. PMID:12223584,
DOI:10.1523/JNEUROSCI.22-18-08297.2002
- 12. Levin MF: Interjoint coordination during pointing
movements is disrupted in spastic hemiparesis. Brain 1996;119:281–293. PMID:8624689,
DOI:10.1093/brain/119.1.281
- 13. Archambault P, Pigeon P, Feldman AG, Levin MF:
Recruitment and sequencing of different degrees of freedom during pointing movements
involving the trunk in healthy and hemiparetic subjects. Exp Brain Res 1999;126:55–67.
PMID:10333007, DOI:10.1007/s002210050716
- 14. Heitz RP: The speed-accuracy tradeoff: history,
physiology, methodology, and behavior. Front Neurosci 2014;8:150. PMID:24966810,
DOI:10.3389/fnins.2014.00150
- 15. Shmuelof L, Krakauer JW, Mazzoni P: How is a
motor skill learned? Change and invariance at the levels of task success and trajectory
control. J Neurophysiol 2012;108:578–594. PMID:22514286,
DOI:10.1152/jn.00856.2011
- 16. Wickelgren WA: Speed-accuracy tradeoff and
information processing dynamics. Acta Psychol (Amst) 1977;41:67–85.
DOI:10.1016/0001-6918(77)90012-9
- 17.Konieczna IM, Deluca TA, Elizabeth A, et al.:
Hoxa10 null animals exhibit reduced platelet biogenesis. 2016.
- 18. Morris DM, Crago JE, DeLuca SC, Pidikiti RD, Taub
E: Constraint-induced movement therapy for motor recovery after stroke.
NeuroRehabilitation 1997;9:29–43. PMID:24526089,
DOI:10.3233/NRE-1997-9104
- 19. Wolf S, Lecraw D, Barton L, Jann B: Forced use of
hemiplegic upper extremities to reverse the effect of learned nonuse among chronic stroke
and head-injured patients. Exp Neurol 1989;104:125–132. PMID:2707361,
DOI:10.1016/S0014-4886(89)80005-6




