2021 Volume 6 Article ID: 20210016
2021 Volume 6 Article ID: 20210016
Background: Congenital limb deficiency is a rare and intractable anomaly of the limbs; however, prostheses can partially complement the motor function and appearance of the missing limbs. The first prosthesis is usually prescribed for children with upper limb deficiencies at approximately 6–8 months of age. In affected children with additional problems associated with motor function, such as limb paralysis, the age for initiating prosthetic therapy and the benefit of prostheses in promoting and expanding their motor function and activities is unknown.
Case: In this case presentation, we describe a 25-month-old boy with cerebral palsy and left unilateral congenital upper limb deficiency caused by congenital constriction band syndrome. The patient could stand with assistance and crawl on his hands and knees. However, he was unable to walk with assistance or to stand on his own. A forearm prosthesis with a passive hand was prescribed and issued, and rehabilitation therapy for wearing and using the prosthesis was performed. At 34 months of age, the patient was able to walk forward using a walker with the prosthesis. Without the prosthesis, he still could not walk using a walker. The upper limb prosthesis also improved other movements such as sitting, standing, and tasks performed on a desk or on the floor.
Discussion: The prosthesis was apparently effective in improving motor function. Prosthesis prescription should be considered at an appropriate and early age considering individual developmental stages and needs, regardless of the existence of additional problems associated with motor function.
Congenital limb deficiency is a rare and intractable anomaly characterised by a reduction or absence of elements in the limbs. The incidence is approximately 4 per 10,000 live births in Japan (2014–2015), among whom the upper limbs are more commonly affected.1) The incidence of congenital upper limb deficiencies is reportedly approximately 3.9–6.2 per 10,000 total births.2,3,4) Congenital limb deficiencies can result from various causes, such as chemicals, pharmaceuticals, infections, metabolic disorders, radiation, and chromosomal or genetic defects.5) Children with congenital limb deficiencies exhibit limitations in activities and are at risk of lower levels of participation in social and leisure activities.6) Patients with congenital limb deficiency require continuous care and support for physical and psychological problems from birth, through childhood and adolescence, to adulthood and beyond. Treatment approaches, including being fitted with an orthosis/prosthesis, surgical treatment (reconstructive surgery or amputation), and rehabilitation therapy, may vary according to the type of deficiency. For example, transverse limb deficiencies are likely to be treated using prostheses in cases of proximal deficiency and using surgery in cases of distal deficiency.7) However, there are no radical treatments for congenital limb deficiency.
Children with upper limb deficiencies are weak in motor skills, and the weakness increases with age.8) In children with unilateral limb deficiencies, gross movements that require equal roles of both upper limbs, such as riding a tricycle or bicycle, are more difficult to achieve than fine movements that require dominant and non-dominant hand functions such as using scissors.8) A prosthesis partially improves the motor function and appearance of the missing limb. It has been reported that the prescription of prostheses and occupational therapy ameliorates children’s motor skill weaknesses.9) In normal practice, the first prosthesis should be prescribed for children with upper limb deficiencies at approximately 6–8 months of age. This is the age at which children without disabilities begin to sit independently with their hands resting on the floor and subsequently without hand support so as to explore and manipulate objects using their hands.10) In Japan, prostheses can be acquired in most cases using the public health system or the social welfare system. However, in children with congenital upper limb deficiencies, prostheses are not sufficiently prescribed, especially when the deficiencies are unilateral.
In children with congenital upper limb deficiencies and additional problems associated with motor function, such as paralysis of limbs, the optimum age for initiating prosthetic therapy and the benefit of prostheses in promoting and expanding their motor function and activities is unknown. This case report describes a child with cerebral palsy and upper limb deficiency caused by constriction band syndrome who was treated effectively with prosthetic intervention and rehabilitation therapy.
The subject matter of this case presentation was approved by the ethics committee of Shizuoka Children’s Hospital (ethical approval number: R1-28). Written consent was not obtained because the child moved away from Shizuoka prefecture and is currently being treated at another hospital. The ethics committee granted approval conditional to the acquisition of oral consent from the guardian. We obtained the necessary oral informed consent from the child’s guardian.
A 25-month-old boy with cerebral palsy and left unilateral congenital upper limb deficiency visited the outpatient clinic of the Department of Rehabilitation Medicine in our paediatric specialty hospital. More specifically, the patient presented with spastic diplegia, i.e., bilateral paralysis in the lower limbs with mild paralysis in both upper limbs. The patient was born at 34 weeks of gestation, with a birth weight of 1996 g, and has bilateral periventricular leukomalacia. The upper limb deficiency occurred as a result of congenital constriction band syndrome; the constriction bands were detected on the left forearm and carpus (Fig. 1a). Surgery for constriction band release was performed when the patient was aged 7 months. The left hand and the distal half of the forearm showed severe hypoplasia. The radius and ulna were present but short; their distal parts were deficient, and skeletal elements were absent in the hand (Fig. 1b). The sequence of his early motor milestones was as follows: head control at 8 months, rolling over at 8 months, sitting without support at 19 months, standing with assistance at 25 months, and crawling on hands and knees at 25 months. The severity of paralysis was graded as level III according to the gross motor function classification system. The skills of walking with assistance and standing on his own were not acquired. There were no signs of co-existing intellectual disability. The patient received physical therapy from 7 months of age for cerebral palsy; however, prescription of a prosthetic and occupational therapy for upper limb deficiency was not instituted. At the first visit, the patient was assessed using the Japanese version of the Vineland Adaptive Behavior Scales, Second Edition,11,12) which is a standardised scale of adaptive behaviour. The adaptive behaviour composite score was 76, whereas the domain standard scores for communication, daily living skills, socialisation, and motor skills were 93, 84, 92, and 52, respectively; the scores are standard scores which have means of 100 and standard deviations of 15. Whereas the patient’s scores for communication and socialisation behaviour were adequate, the scores for motor skills and daily living skills were low and moderately low, respectively. The subdomain scores, called v-scale scores, of motor skills for gross motor and fine motor were 6 and 10, respectively; these scores are standard scores which have means of 15 and standard deviations of 3.

Clinical and radiographic features of the patient. (a) Clinical image depicting the constriction bands detected on the left forearm and carpus (black arrows) and severe hypoplasia of the residual hand. (b) A radiograph of the forearm indicating a deficiency in the distal parts of the left radius and ulna and the absence of skeletal elements of the hand.
The postulated progression in motor function was walking with a hand-held mobility device, such as a posture control walker. Although the patient made attempts to stand, he found it difficult to walk using a walker and to hold on to an object using his hands because of upper limb deficiency. To overcome this problem, an outpatient department at the university hospital that specialises in congenital limb deficiency was consulted, and prosthetic intervention was initiated when the patient was 28 months of age. The patient underwent a standard course of prosthetic intervention for upper limb deficiencies at the university hospital. During the course, the hospital started occupational therapy, and a passive hand prosthesis was prescribed. Occupational therapy was performed once a week for 2 weeks. In general, after 2–3 months, when the children and their guardians have become familiar with the prosthesis, the frequency of therapy is reduced to once every 1–2 months. After 6−12 months, prescriptions of prostheses with voluntary control and the hand, such as myoelectric prostheses, are considered, depending on the children’s needs. For our patient, a forearm prosthesis with a passive hand as the terminal device (Greek Series Infant & Pediatric Hands, TRS, Boulder, CO, USA) was prescribed and issued, and occupational therapy for wearing and using the prosthesis was initiated at the university hospital. In the paediatric hospital, the patient underwent physical therapy, including training for sitting, standing, and walking with the prosthesis, and coaching on daily usage once every 1–2 months. When the patient was aged 29 months, training for walking was instituted using a posture control walker, which was held with the right hand and the left upper limb prosthesis. At first, the physical therapist encouraged and assisted the patient to hold the walker during walking training (Fig. 2). By holding the bar with his left upper limb prosthesis or putting the prosthesis on the bar, the patient could walk using a walker using both upper limbs. The prosthesis was effective for standing and walking. No spatial adjustment of the prosthesis was needed for the child to use the walker. After the patient and his guardians became familiar with the prosthesis, he wore it during the day. The prosthesis was removed at night or when the patient was taking a bath. At 34 months of age, the patient was able to walk forward using the walker in a therapeutic setting, although a therapist was needed to prevent him from falling. Without the prosthesis, the patient could not walk using a walker. The upper limb prosthesis also improved other movements such as sitting, standing, and tasks performed on a desk or on the floor. The patient could put his weight on the prosthetic hand for balance and could hold target objects using the prosthetic hand. When wearing the prosthesis, the patient’s postural balance was more stable, and the quality of tasks performed on a desk or on the floor improved. Task performance was improved by the patient putting his prosthesis on the desk or the floor to maintain a good posture, or by holding the target object with the prosthesis (Fig. 3). At 41 months of age, the patient began trial use of a myoelectric prosthetic hand at the university hospital, and training is currently ongoing. The patient was able to control the open and close operations of the myoelectric hand and was expected to be able to successfully control the myoelectric prosthesis.
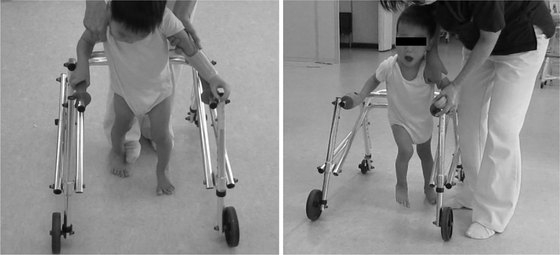
Walking training using a walker. The patient displayed motivation to walk using the walker while wearing his prosthesis during physical therapy. In addition to correction of the lower limb movement, he received assistance from the physical therapist to hold the walker with his prosthesis during the walking exercise.

The patient performed the task of collecting small objects and putting them in a box. (a) Without the prosthesis, the patient’s arm was too short to bear his weight, and postural balance was poor. (b) When the patient wore the prosthesis, he could support himself on the floor using the prosthesis and maintain a good sitting posture. The task could be performed more efficiently when wearing the prosthesis.
The child described in this case presentation showed apparent improvement in gross motor function when using an upper limb prosthesis with a passive hand. Although the patient could not walk using a walker without the prosthesis, he was able to walk using the walker when wearing the prosthesis. He also used the prosthesis effectively for sitting, standing, and performing desk tasks. Children and adolescents with congenital below-elbow deficiencies develop alternatives to wearing prostheses with techniques using body parts such as the stump, head, trunk, mouth, or lower limbs and other creative strategies for daily-life activities.13) However, even cosmetic prostheses are functional in the performance of everyday tasks.14) Indeed, a passive prosthesis was effective and integral for walking with the aid of a walker for the child in this case presentation. To facilitate walking, modification of the walker might have been another option. However, the upper limb prosthesis was effectively used for additional movements such as sitting, standing, and performing desk tasks; in other words, the prosthesis had versatility of function. It is more effective overall to wear a prosthesis than to modify various targeted objects.
The next step of the prosthetic intervention in the current case was a prosthesis with voluntary hand control. In children with unilateral congenital below-the-elbow deficiencies, it has been reported that activities of daily living can often be improved by offering a variety of prosthetic options.15) Hands with voluntary control, such as body-powered or myoelectric prostheses, would be expected to improve the patient’s everyday activities. A myoelectric prosthetic hand is heavier than a passive hand and requires control of muscle contraction. Although the use of myoelectric prosthetic hands may be affected by muscle weakness and abnormal muscle tone caused by cerebral palsy, our patient would be expected to acquire myoelectric prosthesis control. In Japan, prostheses including myoelectric hands can be prescribed by the public health system or the social welfare system. However, the provision of myoelectric prosthesis is rarely permitted. Our patient’s myoelectric prosthesis was not provided by the public health system or the social welfare system. It therefore remains a challenge for our patient to acquire a myoelectric prosthesis.
Prosthetic rejection often occurs during prosthetic intervention. For better acceptance of prostheses, it is recommended that children with congenital limb deficiency start using them when less than 2 years of age because rejection rates are higher among older children.16) Furthermore, children with upper limb deficiencies are reportedly also deficient in visuospatial and lexical-semantic body knowledge of the upper limbs.17) Therefore, to encourage such children to wear, cognise, and use their prostheses as a body part, administering their first prosthesis at an early age is advantageous.
It is difficult to determine the appropriate age at which to initiate prosthetic intervention in children with additional motor function problems. However, in the present case, the prosthesis was apparently effective in improving motor function. Prosthesis prescription should therefore be considered at an appropriate and early age while taking into account individual developmental stages and needs. We believe that providing prostheses and rehabilitation therapy, including prosthetic use training via the public health system, is reasonable and provides meaningful improvement for children with congenital upper limb deficiencies, regardless of the existence of additional problems associated with motor function. In summary, prosthesis prescription should be considered at an appropriate and early age while taking into account individual developmental stages and needs regardless of the existence of additional problems associated with motor function.
For their contributions to this presentation, we would like to acknowledge Certified Prosthetist and Orthotist Teruki Shibata and Tsutomu Echizenya of Tazawa MFG Co. for coordinating prosthesis design and construction. We would like to thank Editage (www.editage.jp) for English language editing.
The authors declare that there are no conflicts of interest.