2021 年 6 巻 論文ID: 20210029
2021 年 6 巻 論文ID: 20210029
Objective: The aim of this study was to demonstrate the non-inferiority of S-flurbiprofen plaster (SFPP) monotherapy for treating knee osteoarthritis compared with the combination of conventional oral and topical non-steroidal anti-inflammatory drugs (NSAIDs).
Methods: A total of 222 participants (114, SFPP group; 108, control group) were treated for 4 weeks. The primary endpoint was the change in the degree of pain felt while rising from a chair after 2 and 4 weeks of treatment as determined using the visual analog scale (VAS) . The secondary endpoint was the change in functional scores and test results. Safety was evaluated in terms of the adverse effects.
Results: The VAS score significantly decreased in both groups after 2 and 4 weeks of treatment. Non-inferiority in the VAS score was established only at 2 weeks. There were no significant differences in the secondary endpoints between the groups. Skin disorders were more frequent in the SFPP group; however, there was no difference in gastrointestinal (GI) adverse effects.
Conclusions: The therapeutic efficacy of SFPP monotherapy for knee OA, with respect to changes in the VAS, was not shown to be non-inferior to conventional treatment at 4 weeks; however, non-inferiority was established at 2 weeks. The functional improvement in the SFPP group was comparable to that of the control group. No severe GI adverse effects associated with SFPP administration were observed; however, it is necessary to pay more attention to the occurrence of skin disorders with SFPP than with conventional topical NSAIDs.
Knee osteoarthritis (OA) is commonly seen in middle-aged and elderly people. It results from the regression of articular structures, such as articular cartilage.1) The main symptoms of knee OA include chronic pain, inflammation, and motor dysfunction; overall, these significantly reduce the quality of life (QOL)2) and constitute a risk factor of locomotive syndrome.3,4) Currently, land-based exercise, weight management, and mind–body exercise are core treatments for knee OA, and core treatments alone or in combination with interventions are recommended as multimodal treatments.5) Pain control is important when performing exercise, and it was revealed that about half of the patients receiving physical therapy used non-steroidal anti-inflammatory drugs (NSAIDs).6) Topical NSAIDs are the most strongly recommended treatment modality according to the latest Osteoarthritis Research Society International (OARSI) guidelines, whereas oral NSAIDs are conditionally recommended.5)
S-Flurbiprofen plaster (SFPP), which contains the primary active NSAID S-flurbiprofen, was launched in Japan in January 2016. S-Flurbiprofen is highly skin permeable and reaches a significantly higher concentration in the synovium than topical flurbiprofen does.7) The usage of SFPP is limited to two patches (total 80 mg/day), and the systemic effects of NSAIDs can be anticipated. Pain relief with SFPP is superior to that obtained with topical flurbiprofen.8) In a previous study on the efficacy of long-term SFPP administration, a marked improvement in the total clinical symptoms score was observed 2–4 weeks after application; moreover, the improvement continued for 52 weeks.9) The major adverse effects observed were skin disorders.9) Furthermore, the incidence of severe gastrointestinal (GI) adverse effects was relatively low; the tolerability of SFPP was confirmed in a previous long-term study.9)
In conventional interventions for knee OA, oral and topical NSAIDs have been used in various combinations. Additionally, the concomitant use of prophylactic medicine for GI adverse effects is required when administrating non-selective NSAIDs.5) The issue of polypharmacy in elderly patients has also become prominent in recent years.10) Considering this social background, SFPP is anticipated to be a beneficial option in the multimodal treatment of knee OA; however, clinical data on SFPP use remain insufficient. Yataba et al.8) conducted a comparative study between SFPP and conventional flurbiprofen patches and concluded that the pain relief was greater in the SFPP group. To the best of our knowledge, no clinical study has compared the efficacy of SFPP monotherapy with that of combined oral and topical NSAIDs. The objective of the current study was to determine whether the efficacy of SFPP monotherapy was non-inferior to that of the combination of conventional oral and topical NSAIDs in patients with knee OA. We hypothesized that the efficacy of SFPP monotherapy would not be inferior to that of combination therapy.
This open-label, multicenter, prospective, randomized study was approved by the Ethics Committee of Hirosaki University (#09H29003). A total of 222 participants with knee OA were enrolled between November 2017 and July 2019 (173 women; 49 men; mean age ± standard deviation (SD), 69.2 ± 9.9 years; mean height ± SD, 155.2 ± 8.6 cm; mean weight ± SD, 60.7 ±11.6 kg). Participants with no pain in the non-target knee were included, and the quality of pain (acute or chronic, severity of inflammation, central sensibilization) was not considered in this study. The injection of hyaluronic acid or steroids was prohibited during the study period. The commencement of new physical therapy during the study period was also prohibited; however, patients who had already started physical therapy when participating in this study were allowed to continue physical therapy. The inclusion and exclusion criteria are shown in Table 1. The patients were randomly assigned in a 1:1 ratio to either the SFPP group or the control group. The randomization factors included gender (male and female), visual analog scale (VAS) score (≥60 and <60 mm), Kellgren-Lawrence grade (II and III), current smoking (yes and no), and body mass index (≥25 and <25 kg/m2). The assignment procedure was performed at the data center, and the attending physicians were blinded to the process. The allocation system used in this study was created by the data center. After obtaining informed consent from the patients, the principal investigator accessed the website for allocation and entered the randomization factors. Allocation was done by the minimization method. The allocation data were stored on a server at the data center that was not accessible by the principal investigator.
| Inclusion criteria | Exclusion criteria |
| · Age ≥20 years | · History of knee arthroplasty in the target knee |
| · Kellgren-Lawrence Grade II or III knee osteoarthritis in the target knee | · Other knee pain or comorbidity (neuropsychiatric diseases, dementia, severe hypertension, peptic ulcer, or skin disease) |
| · No treatment of the target knee osteoarthritis within 14 days prior to the start of the study (medications were allowed if needed) | · History of dermatitis requiring treatment by any patch formulation |
| · VAS score of ≥40 mm at the baseline | · History of allergy to NSAIDs |
| · No pain in the non-target knee | · Aspirin-induced asthma (induction of asthmatic attack by NSAIDs) or a similar experience following treatment with other drugs (enoxacin hydrate, lomefloxacin, norfloxacin, or prulifloxacin) |
| · Pregnant or potentially pregnant women | |
| · Presence of other contraindications as per the product document |
This study was conducted in compliance with the study protocol, the Declaration of Helsinki, and the Ethical Guideline for Clinical Research and the Clinical Trials Act of the Ministry of Health, Labour and Welfare of Japan, with the approval of the Ethics Committee at each study institution. Written consent was obtained from the patients after an appropriate explanation by the physicians.
The patients assigned to the SFPP group had SFPP applied once daily to the affected area, and those assigned to the control group were treated with a combination of oral and topical NSAIDs. The dose of SFPP was not specified in this study design and was determined by the attending physician according to the patient’s symptoms. The oral NSAIDs used in the control group included celecoxib 100 mg twice daily or loxoprofen 60 mg three times daily. NSAID patches (40 mg ketoprofen or 100 mg loxoprofen) were applied to the affected area once daily using tape. The treatment period was set at 4 weeks after referring to previous studies examining the efficacy and safety of other topical NSAIDs including diclofenac11) and eltenac.12) The use of prophylactic medications for gastritis was allowed at the discretion of the physician.
The primary endpoint of this study was the change in VAS scores (0−100 mm) for pain levels while rising from a chair at 2 and 4 weeks after the start of treatment (or at the time of discontinuation). The secondary endpoints included the changes in the Knee Injury and Osteoarthritis Outcome Score (KOOS) for pain,13) the Japanese Orthopaedic Association (JOA) score,14) the two-step test,15) the 25-question Geriatric Locomotive Function Scale (GLFS-25) score,16) and the EuroQOL 5 dimensions 5-level (EQ-5D-5l) score,17) after 2 and 4 weeks of treatment. The safety endpoints were the adverse effects that occurred during the 4-week treatment period and the administration of gastric medications.
The change in VAS score after 2 and 4 weeks was based on a covariant model, with the baseline VAS as a covariate and the treatment group as the independent variable, to calculate the 95% confidence interval of the inter-group adjusted mean (control group minus test group). In a previous study that used SFPP and a placebo, the standard deviation of the change in VAS score was 17.1 mm.8,9) As a principle of statistical analysis, one-third of the standard deviation can be set as the non-inferiority margin if the evaluation value is normally distributed. Therefore, the non-inferiority margin of the VAS score was defined as 6 mm in this study. Consequently, if the upper limit of the inter-group 95% confidence interval is <6 mm, the study drug group is considered to be non-inferior to the control drug group. The other endpoints were compared between the groups using covariance analysis at baseline. The paired t-test was used for comparing the pre- and post-treatment results between the groups. Fisher’s exact test was performed to compare the incidence of adverse effects between the groups and the number of subjects in which prophylactic medications for gastritis were taken orally during the study. A total of 105 subjects were required in each group to statistically demonstrate non-inferiority with a common standard deviation of 15.5 mm, a difference between the control and SFPP groups of 0 mm, a one-sided significance level of 0.025, and a power of detection of 0.8. The single-sided significance level was 2.5% for the primary endpoint and 5% on both sides for the secondary endpoints and safety assessment. All analyses were performed using R version 3.4.0.
In this study, 114 participants (mean age, 68.7 ± 10.2 years) were assigned to the SFPP group and 108 (mean age, 69.7 ± 9.5 years) were assigned to the control group (Table 2). A total of 28 participants dropped out during the investigation period for the following reasons: patient’s decision (n=3), adverse events (n=14), discontinuation of study treatment (n=4), physician’s judgment (n=3), and others (n=5) (including duplications) (Fig. 1).
| SFPP group (n=114) |
Control group (n=108) |
|
| Age [years] | 68.7 ± 10.2 | 69.7 ± 9.5 |
| Sex (male:female) | 25:89 | 24:84 |
| Height [cm] | 154.9 ± 7.7 | 155.5 ± 9.5 |
| Body weight [kg] | 60.7 ± 11.2 | 60.7 ± 12.1 |
| BMI [kg/m2] | 25.2 ± 4.0 | 25.0 ± 3.6 |
| Disease duration [days] | 581.8 ± 1388.4 | 610 ± 1400.1 |
| Comorbidity (n) | 60 | 50 |
| Gastrointestinal disorders | ||
| Gastritis | 4 | 2 |
| Others | 6 | 5 |
| Cardiovascular diseases | ||
| Hypertension | 39 | 39 |
| Arrhythmia | 2 | 1 |
| Ischemic heart disease | 0 | 1 |
| Others | 1 | 0 |
| Chronic kidney disease | 0 | 1 |
| Metabolic endocrine disease | ||
| Diabetes mellitus | 10 | 7 |
| Others | 17 | 13 |
The data are mean ± SD or n.
BMI: body mass index.
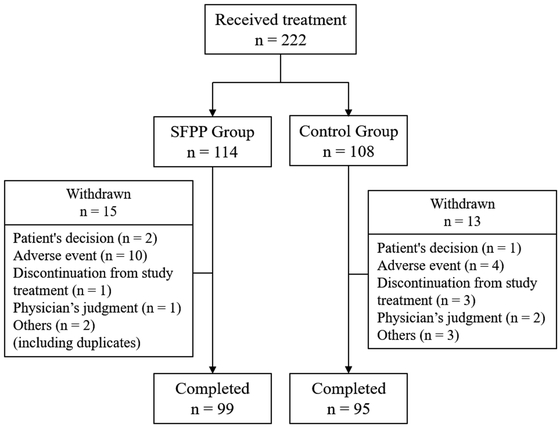
Flow diagram for study participants.
In the SFPP group, the VAS score changed from 58.3 ± 13.8 mm to 28.3 ± 22.5 mm at 2 weeks (P<0.001) and to 26.4 ± 23.2 mm at 4 weeks (P<0.001). In the control group, the VAS score changed from 59.6 ± 14.4 mm to 31.2 ± 20.9 mm at 2 weeks (P<0.001) and to 20.7 ± 18.4 mm at 4 weeks (P<0.001) (Figs. 2 and 3). The change in VAS score was determined using a covariate model with the VAS score at baseline, which resulted in a change of −30.5 mm in the SFPP group and −28.0 mm in the control group after 2 weeks, and −32.4 mm in the SFPP group and −38.3 mm in the control group after 4 weeks (Table 3). After 2 weeks, the mean difference was −2.4 mm with a 95% confidence interval of −8.4 to 3.6 mm. The upper limit of the 95% confidence interval did not exceed the non-inferiority margin of 6 mm, and non-inferiority was therefore established. However, after 4 weeks, the mean difference in the VAS score of 5.9 mm yielded a 95% confidence interval of 0.1–11.7 mm and, consequently, non-inferiority was not established (Table 3).
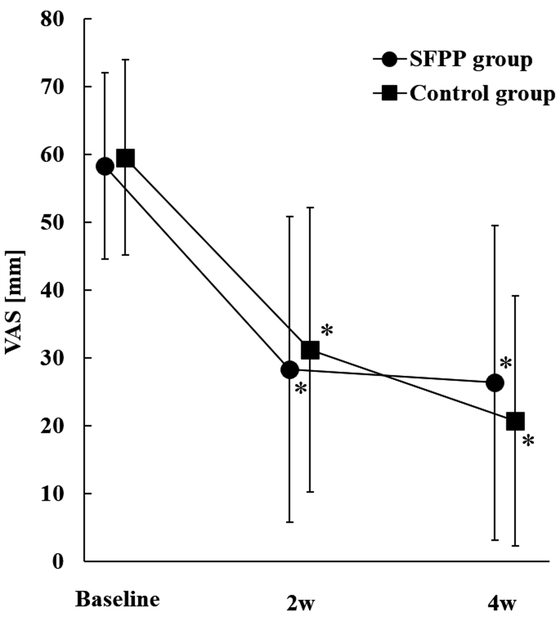
Temporal change in VAS scores in both groups. *P<0.05 (vs. baseline), by paired t-test.

Amount of change in VAS scores in both groups.
| Baseline | 2 weeks | 4 weeks | ||||
| SFPP group (n=114) |
Control group (n=107) |
SFPP group (n=99) |
Control group (n=91) |
SFPP group (n=107) |
Control group (n=97) |
|
| VAS [mm] | 58.3 ± 13.8 | 59.6 ± 14.4 | 28.3 ± 22.5* | 31.2 ± 20.9* | 26.4 ± 23.2* | 20.7 ± 18.4* |
| Magnitude of change (covariate model) [mm] | – | – | –30.5 | –28.0 | –32.4 | –38.3 |
| Difference in the mean change (95% CI) [mm] | – | –2.4 (−8.4, 3.6) |
5.9 (0.1, 11.7) |
|||
The data are mean ± SD.
*P<0.001 (paired t-test vs. baseline).
After treatment, the KOOS-Pain score, JOA score, two-step test, GLFS-25 score, and the EQ-5D-5l score changed significantly in both the SFPP and control groups (Table 4). There was no significant difference in the size of the change in the KOOS-Pain score, JOA score, two-step test, GLFS-25, and EQ-5D-5l between the groups (Fig. 4).
| SFPP group | Control group | |
| KOOS-Pain | ||
| Baseline | 56.9 ± 14.9 | 54.3 ± 15.5 |
| 2 weeks | 68.1 ± 16.8* | 64.9 ± 14.6* |
| 4 weeks | 71.3 ± 16.2* | 71.9 ± 14.3* |
| JOA score | ||
| Baseline | 74.7 ± 12.9 | 73.9 ± 12.9 |
| 2 weeks | 82.9 ± 14.6* | 81.7 ± 12.5* |
| 4 weeks | 85.8 ± 15.0* | 85.1 ± 11.7* |
| Two-step test | ||
| Baseline | 1.01 ± 0.21 | 0.96 ± 0.21 |
| 2 weeks | 1.06 ± 0.23* | 1.03 ± 0.22* |
| 4 weeks | 1.11 ± 0.24* | 1.06 ± 0.22* |
| GLFS-25 | ||
| Baseline | 23.2 ± 14.1 | 25.9 ± 15.8 |
| 4 weeks | 16.3 ± 13.7* | 16.8 ± 12.8* |
| EQ-5D-5l | ||
| Baseline | 0.74 ± 0.14 | 0.71 ± 0.15 |
| 4 weeks | 0.81 ± 0.12* | 0.82 ± 0.12* |
The data are mean ± SD.
*P<0.001 (paired t-test vs. baseline).
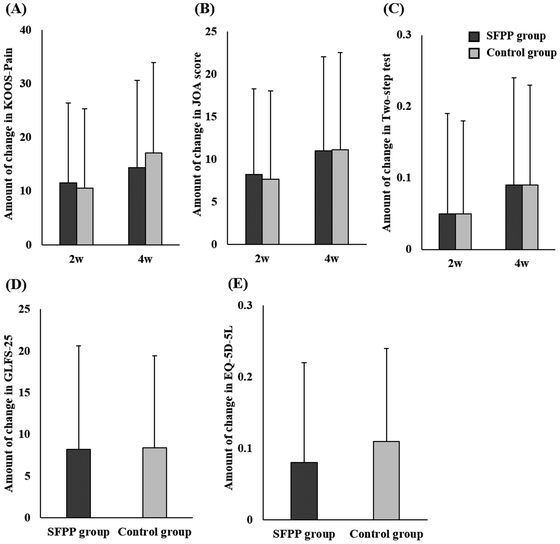
The size of the change in each secondary endpoint in both groups. (A) KOOS-Pain, (B) JOA score, (C) two-step test, (D) GLFS-25, (E) EQ-5D-5l (KOOS, Knee Injury and Osteoarthritis Outcome Score; JOA, Japanese Orthopaedic Association; GLFS, Geriatric Locomotive Function Scale, EQ-5D-5l, EuroQOL 5 dimensions 5-levels).
A total of 31 adverse events occurred during the study period (25 in the SFPP group, and 6 in the control group) (Table 5). The incidence of adverse events was significantly higher in the SFPP group (P<0.001). The main adverse events noted in the SFPP group were skin disorders, such as dermatitis, erythema, and eczema. The incidence of these adverse events was significantly higher in the SFPP group than in the control group (P<0.001). Three GI adverse effects in the SFPP group and two in the control group were noted, with no significant difference between the groups (P=1.000).
| Total (n=222) |
SFPP group (n=114) |
Control group (n=108) |
P-value | |
| Total number of adverse events | 31 (14.0) | 25 (21.9) | 6 (5.6) | <0.001 |
| Skin disorders | <0.001 | |||
| Dermatitis | 7 (3.2) | 7 (6.1) | – | |
| Erythema | 6 (2.7) | 4 (3.5) | 2 (1.9) | |
| Eczema | 4 (1.8) | 4 (3.5) | – | |
| Itching | 3 (1.4) | 2 (1.8) | 1 (0.9) | |
| Contact dermatitis | 3 (1.4) | 3 (2.6) | – | |
| Gastrointestinal disorders | 1.000 | |||
| Abdominal discomfort | 3 (1.4) | 1 (0.9) | 2 (1.9) | |
| Nausea | 2 (0.9) | 2 (1.8) | – | |
| Oral ulcer | 2 (0.9) | 1 (0.9) | 1 (0.9) | 1.000 |
| Palpebral swelling | 1 (0.5) | 1 (0.9) | – | 1.000 |
The data show the number of cases (%).
Medication for gastritis was administered prophylactically at the start of treatment for none of the patients in the SFPP group and for 25 patients in the control group (P<0.001). Medications for gastritis were prescribed prophylactically or therapeutically for adverse events for 5 patients in the SFPP group and 39 patients in the control group (P<0.001) at 2 weeks, and for 4 patients in the SFPP group and 34 patients in the control group (P<0.001) at 4 weeks (Table 6).
| Baseline | 2 weeks | 4 weeks | |||||||
| SFPP group (n=114) |
Control group (n=108) |
P-value | SFPP group (n=112) |
Control group (n=101) |
P-value | SFPP group (n=100) |
Control group (n=96) |
P-value | |
| Total number of uses of medication | 0 | 25 (23.1) | <0.001 | 5 (4.5) | 39 (38.6) | <0.001 | 4 (4.0) | 34 (35.4) | <0.001 |
| Preventive administration | 0 | 25 (23.1) | <0.001 | 4 (3.6) | 37 (36.6) | <0.001 | 3 (3.0) | 32 (33.3) | <0.001 |
| Gastric coating agent | 0 | 19 | 1 | 30 | 0 | 28 | |||
| PPI | 0 | 5 | 2 | 7 | 2 | 5 | |||
| H2-blocker | 0 | 1 | 0 | 2 | 0 | 1 | |||
| Acid suppressant | 0 | 0 | 0 | 0 | 1 | 0 | |||
| Others | 0 | 0 | 1 | 2 | 0 | 2 | |||
| Treatment of gastrointestinal adverse event | – | – | – | 1 (0.9) | 2 (2.0) | 0.605 | 1 (1.0) | 2 (2.1) | 0.615 |
| Gastric coating agent | – | – | 1 | 1 | 1 | 1 | |||
| PPI | – | – | 0 | 1 | 0 | 1 | |||
| H2-blocker | – | – | 0 | 0 | 0 | 0 | |||
| Acid suppressant | – | – | 0 | 0 | 0 | 0 | |||
| Others | – | – | 0 | 0 | 0 | 0 | |||
The data show the number of cases (%).
Fisher's exact test was performed.
Some subjects were prescribed more than one of medicine.
H2 blocker: histamine type 2 receptor antagonist.
In this study, the treatment efficacy of SFPP monotherapy was found not to be non-inferior to conventional treatment at 4 weeks following the initiation of treatment. However, VAS score results within the non-inferiority margin were exhibited at 2 weeks, and there were no significant differences in the secondary endpoints between the two groups. The incidence of skin disorders was significantly higher in the SFPP group; in contrast, the incidence of GI adverse effects in the SFPP group (without prophylactic administration) was similar to that in the control group.
Yataba et al.9) demonstrated that the number of patients with marked improvement continuously increased from 2 to 52 weeks after SFPP application in the patients’ global assessment. Moreover, their study suggested that the improvement in the clinical symptom score was apparent in the early phase, especially during the first 2 weeks. In a phase 3 randomized controlled trial, the superiority of SFPP to conventional topical NSAIDs, in terms of efficacy, was demonstrated.8) The improvement in VAS scores following rising from a chair was 40.9 mm in the SFPP group and 30.6 mm in the flurbiprofen patch group, the difference being significantly higher in the SFPP group.8) Considering the recommendation of the OARSI guideline,5) SFPP may play a crucial role as an initial treatment for knee OA because its non-inferiority was established at 2 weeks in this study, but not at 4 weeks.
The incidence of skin disorders was 17.5% in the SFPP group and 2.8% in the control group. In a previous study investigating the safety of SFPP, drug-related skin symptoms were observed in 46.8% of application sites.9) A systematic review demonstrated that adverse effects of conventional topical NSAIDs on the application site were found in up to 39.3% patients.18) In the current study, skin disorders were observed in 17.5% of patients in the SFPP group. Although it has been suggested that topical NSAIDs are relatively effective and safe for OA,19) skin disorders cannot be ignored from the viewpoint of compliance. Physicians should be aware of the incidence of skin disorders and obtain informed consent from the patient when treating OA with SFPP.
Although it is well known that oral NSAIDs display a superior effect on pain, the greatest challenges with their administration are the numerous adverse effects, such as GI symptoms,20) cardiovascular diseases,21) and renal dysfunction.22) Because the absorption of oral drugs increases the incidence of these adverse effects, the OARSI guidelines recommend using non-selective NSAIDs with a proton pump inhibitor (PPI) or a COX-2 inhibitor while administering oral NSAIDs.5) A systematic review by Sardana et al.23) examined 3619 patients with knee OA and found that topical ketoprofen was associated with fewer adverse GI events than oral celecoxib and had a similar frequency of adverse GI events to that of a topical placebo. Yataba et al.9) reported the incidence of GI adverse events as 3 in 101 patients treated with SFPP 40 mg/day and 9 in 100 patients treated with SFPP 80 mg/day over the 52-week treatment period. They also reported no cardiovascular complications associated with SFPP. Regarding renal function, no clinically significant increase compared to the baseline in blood urea nitrogen or creatinine was reported after the start of treatment.9,24) In the current study, GI adverse events were confirmed at a rate of 2.6% in the SFPP group and 1.9% in the control group, with no significant difference. The combination of prophylactic medications for gastritis was allowed in this study design. In fact, significantly more patients were administered with prophylactic medications in the control group than in the SFPP group. Consequently, these results suggested that the risk of GI adverse events during treatment with SFPP was just as low as, if not lower than, that with conventional treatment.
This study had some limitations. First, the therapeutic agents used in the control group were not uniform, and the use of prophylactic medication for gastritis was allowed at the discretion of the physician. The OARSI guideline recommends the use of non-selective NSAIDs, preferably with the addition of a proton pump inhibitor, or a selective COX-2 inhibitor as a conditional recommendation (Level 1B).5) In clinical practice, the type of NSAID is selected according to the patient characteristics or comorbidities. Similarly, the prophylactic prescription of anti-gastritis medication could differ among the attending physicians. This multicenter study was designed to be generalized to actual clinical practice by comparing the efficacy of SFPP and conventional NSAIDs, which many surgeons currently prescribe. Therefore, the choice of NSAID and prophylactic administration for gastritis was flexible in this study. Second, the dose of SPFF was not specified. When 80 mg of SFPP was applied, the dose equaled that of an oral flurbiprofen preparation. To evaluate the systemic analgesic effect of SFPP absorbed through the skin, it may have been better to standardize the dose of SFPP at 80 mg/day. However, this study was designed so that the therapeutic effects could be examined by using drug administration methods that were as close as possible to the actual clinical situation. This approach was taken because the analgesic effect of SFPP might have been overestimated if the dose of SFPP specified as 80 mg/day did not match actual clinical situation. Third, physical activity, muscle mass, and the walking ability of the patients at baseline were not collected in this study. Differences in levels of physical activity might influence the objective and subjective outcomes of the treatment for knee OA patients. Fourth, the application time of topical NSAIDs might affect the incidence of skin adverse events. Although the SFPP group demonstrated higher numbers of skin adverse events, the application time of the compress could not be investigated in this study and was left to principal physicians to decide. Finally, the treatment intervention period was only 4 weeks. Previously, the analgesic effects of SFPP were observed early after administration.8) However, because our results revealed that the incidence of skin disorders was relatively high, it was considered that a longer-term clinical study would be necessary to evaluate the tolerability and safety of SFPP treatment.
The therapeutic efficacy of SFPP monotherapy in knee OA was not shown to be non-inferior to conventional treatment at 4 weeks; however, its non-inferiority was established at 2 weeks with respect to changes in the VAS score following rising from a chair. The functional improvements in the SFPP group were comparable to those in the control group. No severe GI adverse effects associated with SFPP administration were observed; however, it will be necessary to pay more attention to the occurrence of skin disorders with SFPP than with conventional topical NSAIDs.
We thank the following facilities for their participation in the study: Aomori Rosai Hospital, Odate Municipal General Hospital, Japan Community Health Care Organization Akita Hospital, Hirosaki Municipal Hospital, Hirosaki National Hospital, Kuroishi General Hospital, Towada Municipal Central Hospital, Noheji General Hospital, Kishiya Seikei−Geka Clinic Murakami Hospital, Yokoyama Orthopedic Clinic, Takamori Orthopedics, Internal Medicine and Dental Clinic Kobose Hospital, Ikeda Kinen Hospital, Chihaya Hospital, Yoshimura Orthopedic Clinic, Urata Orthopedic Clinic, Nanko Hospital, Koenji Orthopedic Clinic, Machiya Orthopedic Clinic, Matsumoto Hospital, Ozawa Orthopedic Clinic, and Sannomiya Clinic.
Yasuyuki Ishibashi is on the speakers’ bureaus for Taisho Pharmaceutical Co., Ltd. and Teijin Pharma Ltd. The other authors declare that there are no conflicts of interest.