2024 Volume 12 Pages 1-12
2024 Volume 12 Pages 1-12
Xylooligosaccharides are functional food supplements that refer to indigestible oligosaccharides consisting of D-xylose molecules linked by β-1,4-glycosidic bonds. Xylooligosaccharides in the human body perform the functions of prebiotics; they have antioxidant, immunomodulatory and antitumor effects. In industry, xylooligosaccharides are obtained from lignocellulosic biomass by hydrolysis of the xylan contained therein. Chemical hydrolysis is carried out with acid or alkali solutions and is associated with negative environmental consequences. Autohydrolysis refers to methods of physical impact on lignocellulosic raw materials, in which, under the action of high pressure and temperature, steam cracking of raw materials and separation of carbohydrates into fractions occur. Enzymatic hydrolysis of xylan provides the purest products and allows you to control the functional orientation of the resulting xylooligosaccharides. Hydrolysis of xylan to obtain xylooligosaccharides is carried out by microbial xylanases and β-xylosidases produced by bacteria, molds, and actinomycetes. Xylanase producers are ubiquitous in lignocellulosic raw materials and isolated from soil, corn cobs, wheat husks, rice and wheat straw, sugarcane bagasse, oil palm waste, wood chips, sugar bagasse, forest litter, and eucalyptus leaves.
Oligosaccharides are inherently polymers containing from two to ten monosaccharide residues. Due to the sweet taste, oligosaccharides act as sweeteners in the composition of food products and also affect their consistency, maintaining the structure of the products. Many oligosaccharides are non-digestible carbohydrates; therefore, they can act as substrates for beneficial microflora inhabiting the intestinal tract of humans and animals (lactobacilli, bifidobacteria) and that is, they function as prebiotics [1]. In addition to the prebiotic action, oligosaccharides exhibit pharmacological activity, for example, inulin helps to reduce blood sugar and is the most important supplement for the nutrition of diabetic patients. Recently, there has been a widespread increase in the focus on healthy eating, consumers more often prefer functional foods, which leads to interest in new sources of prebiotic supplements. Xylooligosaccharides (XOS) are relatively new promising nutrients for a healthy diet.
XOS is a mixture of oligosaccharides such as xylobiose, xylotriose, and xylotetrose, which consist of D-xylose molecules connected by β-1,4-glycosidic bonds [2, 3]. The structure of XOS, namely the presence of β-1,4-glycosidic bonds, determines their high acid stability, due to which they are protected from decomposition when passing through the acidic environment of the stomach and enter the intestine unchanged, where they are utilized by the intestinal microflora. Anti-inflammatory, antioxidant, antitumor, and antimicrobial effects of this group of oligosaccharides have also been established. XOS is stable at pH 2.5 to 8, does not degrade at 100 °C and definitely has an advantage over other oligosaccharides, such as fructooligosaccharides, as they can be included in any technological food production processes, including the manufacture of sterilized products, the production of fruit juices with low pH. Another advantage of XOS is that they exhibit their pharmacological effects in a lower dosage compared to fructooligosaccharides (0.7 g per day instead of 3.0 g), as it minimizes the development of side effects when they are used [4, 5].
Small amounts of XOS are found in vegetables, fruits, grains, honey, and milk. In an industrially significant amount, XOS is obtained by the hydrolytic decomposition of xylan, the main representative of hemicelluloses found in plant cell walls [6]. Since a rich source of xylan is the secondary raw vegetable material of processing enterprises, XOS can be considered a high-value-added product. This review considers various sources and approaches to the technology of obtaining XOS using chemical, physical, and microbiological methods.
Xylan, as well as cellulose, is the main component of plant cell walls; its mass fraction is up to 30% of the dry matter of the cell. Xylan is ubiquitous in terrestrial annual plants, deciduous and coniferous plants, mosses, and ferns and is synthesized by seaweeds. Mostly, xylans are components of plant tissues that perform structural functions but are also present in the walls of growing cells, seeds, and bulbs of certain plant species [7].
Xylans form a strong heterogeneous structure built by joining cellulose fibers into microfibrils. Unlike cellulose, xylans have shorter (500-3000 D-xylose residues) and branched chains. Depending on the nature of the branches, xylans are divided into arabinoxylans, glucuronoxylans, and arabinoglucuronoxylans. In arabinoxylans, L-arabinofuranose could be attached onto C-2, C-3, or both atoms of the D-xylose residue. Glucuronoxylans contain D-glucuronic acid (or its derivative 4-O-methyl-D-glucuronic acid), which binds to the C-2 positions of the D-xylose residue. Arabinoglucuronoxylans are xylans containing both sugars: L-arabinofuranose and D-glucuronic acid. D-xylose residues in xylans can also be replaced by ferulic acid, p-coumaric acid, or an acetyl group. The degree of branching and the composition of the xylan molecule differ for types of plant materials. Thus, the presence of arabinoxylan is typical for cereal crops, glucuronoxylans are the main hemicellulose components of hardwoods (birch and poplar), and glucuronoarabinoxylans are found in herbs and cereals [5, 8, 9]. Heteroxylans containing various mono- and oligosaccharide residues are found in cereal bran, seeds, and gum exudates [5].
In plant raw materials, xylan is associated with other components of the cell wall, such as cellulose and lignin. If the bond between xylan and cellulose is due to weak hydrogen interactions, then lignin forms strong ester bonds with xylan, the latter is a problem in the extraction of xylan from plant materials and its hydrolysis [8, 9].
Despite the fact that attention to XOS as prebiotic nutritional components has arisen recently, the first attempt to obtain them from lignocellulosic raw materials was made in 1984 when studying the decomposition of plant oligomers [10]. Today, the traditional source of xylan for XOS production is the plant mass of agro-industrial waste (lignocellulosic biomass). Most XOS today are produced by the hydrolysis of corn cob xylan. Commercially promising sources are cereal straw, sorghum, bagasse, corn stalks and husks, flax shavings, wheat bran, almond shells, and bamboo [9, 11].
Thus, the degradation of plant xylan is a traditional way to obtain XOS. Since lignocellulosic raw materials of various origins contain xylans differing in chemical structure, the choice of biomass for degradation can determine the structure and properties of the resulting XOS [5, 8, 9]. The structure of chemical bonds between xylan and other components of the plant cell causes the main difficulties in obtaining XOS from agricultural waste [8, 9]. In the next section, various approaches to the processing of lignocellulosic biomass are considered and classified depending on the nature of the factor leading to the destruction of the biomass.
There are several approaches to splitting xylan into XOS, which happen due to chemical and enzymatic hydrolysis and their combination, as well as a physical method of obtaining XOS - autohydrolysis at high pressure and temperature [12, 13].
3.1 Chemical hydrolysis of xylanThe chemical method for obtaining xylooligosaccharides is realized by acid or alkaline degradation of xylan. For the hydrolysis of xylan, inorganic acids such as tartaric, acetic, hydrochloric, and sulfuric acids can be used, while the yield and properties of XOS depend on the concentration of the acid and the duration of hydrolysis. Hydrolysis with tartaric acid yielded xylooligosaccharides from tobacco stem xylan [14]. The experience of obtaining XOS from corn cobs by exposing raw materials to a weak solution of sulfuric acid for 30 minutes at 90 °C is described [15]. The high efficiency of xylan hydrolysis for the isolation of XOS using acetic acid has been established. Zhang H. and co-authors reported that when processing lignocellulosic waste with 20% acetic acid at 140 °C for 20 minutes, the XOS yield exceeded 45.86%, while the use of acetic acid was more efficient in comparison with hydrolysis with sulfuric and hydrochloric acids [16, 17].
Acid hydrolysis requires the use of harsh chemicals and is not environmentally friendly. In addition, the use of acid treatment is limited by the formation of undesirable by-products of carbohydrate hydrolysis, such as furfural, hydroxymethylfurfural, and acetic acid.
Hemicelluloses respond well to alkaline hydrolysis. The efficiency of such hydrolysis increases with increasing alkali concentration. The experience of alkaline hydrolysis of hemicelluloses of pineapple peel and corn cobs was described [15, 17, 18]. Alkaline hydrolysis methods are not widely used in the industrial hydrolysis of lignocellulosic biomass since they can cause equipment corrosion [6].
3.2 AutohydrolysisAutohydrolysis or autohydrolysis-explosion is a steam cracking lasting from several seconds to several minutes, which is carried out at certain pressure and temperature values (100–375 °C, 2.2–6.2 MPa). Initially, wet lignocellulosic raw materials are processed at elevated pressure; afterward, the pressure is abruptly released, which causes the effect of a steam explosion. In this case, the biomass is fractionated into lignin, hemicelluloses, and cellulose [19].
During autohydrolysis, water is ionized, and hydronium ions are formed, which cause catalytic depolymerization of xylan to form xylooligosaccharides. Additional hydronium ions are formed during the destruction of xylan acetyl groups to acetic acid. In this case, cellulose and lignin accompanying xylan remain in the solid phase of the mixture. By this method, XOS was obtained from corn cobs, wheat straw, rice husks, barley straw, almond shells, oil palm leaves, and miscanthus. This method has an undoubted advantage due to the environmental friendliness and purity of the products obtained; however, it is associated with work with complex expensive equipment and is characterized by high energy consumption. With an overall low yield of XOS, the autohydrolysis method has low profitability [10, 13, 20, 21].
3.3 Enzymatic hydrolysis of xylanEnzymatic hydrolysis of xylan proceeds under mild conditions, is environmentally friendly, and makes it possible to obtain XOS of the required structure [22]. Difficulties in the enzymatic production of XOS are due to the presence of lignin and cellulose in the cell walls of plant materials. Lignin, which is associated with hemicelluloses in the composition of the plant cell wall, is a kind of barrier from physical and chemical influences and prevents the enzymatic hydrolysis of raw materials. Therefore, in practice, the preliminary preparation of lignocellulosic biomass is sometimes used to destroy the lignin-hemicellulose complex and enzymatic hydrolysis of xylan to obtain XOS [13]. The effectiveness of using steam, thermal treatment, acid and alkaline hydrolysis, ultrasound, and microwave treatment has been shown as pretreatment [9].
The most preferable method is the lignocellulosic biomass treatment with an alkali solution. Such treatment causes swelling of the structural components of the plant cell, leading to an increase in the area of the inner surface, a decrease in the degree of polymerization, separation of structural bonds between lignin and carbohydrates, and destruction of lignin. Due to this, xylan is easily extracted from the lignin-hemicellulose complex. Studies of the pretreatment of corn cob biomass have shown that exposure to an alkali solution is the most preferable in comparison with the use of acid solutions or sodium hypochlorite and provides a high yield of xylan from lignocellulosic biomass [23].
Hydrolysis of xylan is carried out by two types of enzymes: xylanases and β-xylosidases. The scheme of enzymatic hydrolysis of xylan is shown in Figure 1. Xylanases are endoenzymes and act on the xylose backbone by hydrolyzing β-1,4-glycosidic bonds to form oligosaccharides of various lengths, including xylose and XOS, such as xylobiose. Xylanases belong to the class of GH glycoside hydrolases and include various families (GH2, GH10, GH11, GH16, GH26, GH30, GH31, GH39, GH42, GH43, GH53) [24]. β-xylosidases are exoenzymes and facilitate xylan hydrolysis by cleaving xylose monomers and small XOS, removing side chain substituents from the non-reducing end of the molecule to form xylose [24].
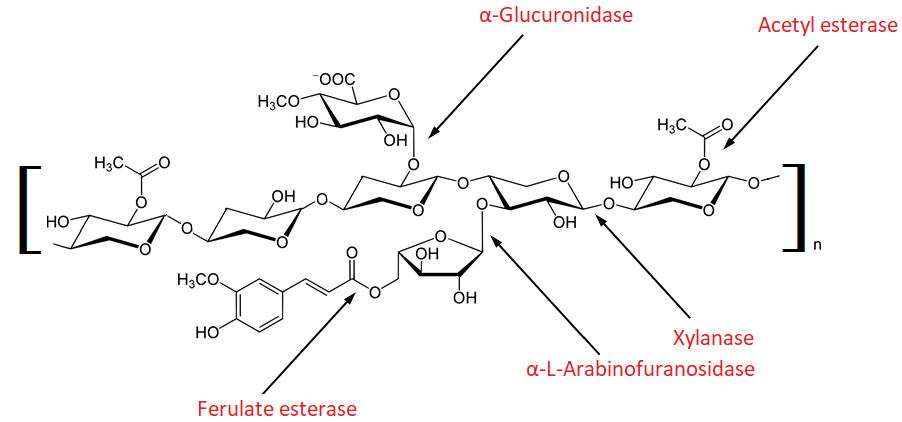
Figure 1: Enzymatic hydrolysis of xylan [1]
The described structure of xylan determines the complexity of its complete enzymatic hydrolysis; therefore, for this purpose, the complex action of various enzymes is often used. α-L-arabinofuranosidases, α-D-glucuronidases, acetylxylanesterases, ferulic acid esterases and p-coumaric acid esterases act on various substituents of the branched xylan molecule, thereby increasing the availability of the xylose backbone for the action of xylanases [24, 25, 26]. It was shown that the yield of XOS during the combined destruction of lignocellulosic biomass by xylanase and arabinofuranosidase was 95.03% versus 30% when using single enzymes [9].
The most studied glycoside hydrolases are those of the GH10 and GH11 families. They are often used to obtain XOS due to their high specificity for xylan [27, 28]. The family GH10 includes endo-1,4-β-xylanases and endo-1,3-β-xylanases; most part of the family members exhibit endo-1,4-β-xylanase activity. The hydrolases of this family are not entirely xylan specific, as they are also capable of targeting low molecular weight cellulose-derived substrates such as aryl cellobiosides and some cellooligosaccharides. In the case of xylan, they hydrolyze not only linear substrates, but also substituted xylans and XOS [27].
The xylanases of the GH11 family are also called “true xylanases” because they are active only on D-xylose-containing substrates and are unable to hydrolyze cellulose-derived substrates. Endo-1,4-β-xylanase and exo-1,4-β-xylosidase activities were found in this family. The main products formed after hydrolysis by these enzymes are xylobiose and xylotriose. The GH11 xylanases hydrolyze the unsubstituted portions of the xylan backbone. They cannot attack the xylose bond next to the branched xylose, towards the non-reducing end. The xylanases of this family are most active against long-chain XOS. Products obtained after the action of GH11 xylanases can be further hydrolyzed by GH10 xylanases [27, 29].
When using xylanases of various families, it is possible to obtain xylooligosaccharides with the desired structure and properties. The use of xylanases of the GH10 family is suitable for the preparation of substituted XOS, since these glycoside hydrolases allow a wider range of side groups and have lower selectivity [30]. The action of endoxylanases of the GH5 family, hydrolyzing xylans with a high degree of substitution by arabinose, makes it possible to obtain arabinoxylooligosaccharides (AXOS). Using the same principle, it is possible to obtain XOS substituted with uronic acid (UXOS) using xylanases of the G30 family. It has been observed that linear XOS and AXOS are fermented by intestinal tract microorganisms faster than UXOS. In addition, comparatively more intestinal bacteria can grow on XOS, while AXOS and UXOS can be used by fewer strains. It has been established that the presence of various types of substituents determines the antioxidant activity exhibited by XOS [29, 30]. Thus, when using xylanases of various families, it becomes possible to selectively obtain prebiotics of the required physiological orientation [29].
Although xylan is amenable to chemical hydrolysis with acids and bases, such approaches to the production of XOS should not be widely used because they cause significant environmental damage [6, 14]. Innovative methods for the destruction of lignocellulosic biomass, based on the use of high pressure and temperature, are associated with the use of expensive equipment and are characterized by low profitability [20, 21]. Enzymatic hydrolysis of xylan is the most environmentally friendly and cost-effective way to obtain XOS, allowing obtaining oligosaccharides of the desired structure. Despite the complexity of enzymatic hydrolysis in the absence of chemical or physical pre-treatment of lignocellulosic biomass, the use of only one-step biotechnological approach is the preferred direction in XOS technology, whereas it let implement an energy-saving, environmentally friendly process. For this approach, it is important to create compositions of enzymes that most completely hydrolyze xylan, providing a high yield of XOS. Such compositions should include xylanases of the GH10 and GH11 families and can be obtained biotechnologically by cultivating bacteria or microscopic fungi.
Many fungi, bacteria, yeasts, actinomycetes and algae are capable of synthesizing enzymes that convert xylan to form xylooligosaccharides. Xylanases have been found in protozoa, crustaceans, snails, insects, in germinating seeds of cucumber and barley, and in the rumen of ruminants. Industrially significant sources of xylanases are fungi, yeasts, and bacteria [24, 31].
Among bacteria, xylanase producers are Arthrobacter, Geobacillus, Pediococcus, Bacillus, Clostridium, Dictyoglomus, Paenibacillus, Rhodothermus, Staphylococcus, Pseudomonas, Thermoactinomyces, Thermotoga [31]. Many bacterial xylanases are highly stable, stable in alkaline and acidic media, in a wide temperature range [31]. Bacteria of the genus Bacillus are known as the most significant sources of xylanases: Bacillus sp., B. pumilus, B. halodurans, B. subtilis, B. amyloliquefaciens, B. circulans and B. stearothermophilus. The production of a thermostable xylanase from a thermophilic B. subtilis strain has been reported; the B. circulans strain was able to produce extracellular xylanase under optimal cultivation conditions [32].
Xylanases have been isolated from microscopic fungi of the genera Talaromyces, Thermomyces, Thermoascus, Melanocarpus, Chaetomium, Fusarium, Humicola, Paecylomyces, Scytalidium, Thielavia, Corynascus, Myceliophthora, Sporotrichum, Rhizomucor, Trichoderma, Aspergillus. For the industrial synthesis of xylanases, the most commonly used fungi are Aspergillus and Trichoderma, as their xylanases have been studied most fully [33, 34]. Less studied but also active producers of xylanolytic enzymes are fungi of the genus Penicillium. It is known about the isolation of hyperthermophilic xylanases from the fungi Thielavia terrestris, Talaromyces thermophilus, Paecilomyces thermophila, Chaetomium sp. X2-8, Rhizomucor pusillus, Rasamsonia emersonii, Aspergillus oryzae, etc. [31].
Xylanases obtained with the help of mold fungi are very diverse, but they are less stable in acidic and alkaline environments; among them, thermophilic and hyperthermophilic forms, active at temperatures above 60 °C, are less common. In large-scale cultivation of mold fungi, their non-pathogenicity is an advantage; in addition, most molds are characterized by the excretion of enzymes into the culture medium, which facilitates the industrial process of their production and allows for maintaining high xylanolytic activity. The difficulty in their cultivation is the conduct of the process in an acidic environment, at an optimal pH for most molds [26, 33].
Thus, bacteria, microscopic fungi, some actinomycetes, and algae are capable of producing xylanolytic enzymes. Xylanases obtained from various microbiological sources differ in activity, specificity, and thermal and acid resistance [24, 31, 32, 33]. To obtain xylanases with the desired properties, it is necessary to isolate the strains producing them from lignin-containing natural raw materials, the sources of which are discussed below.
The production of cellulases and xylanases in microorganisms usually begins when the appropriate substrate is present in abundance in their environment. Thus, many strains producing cellulase and xylanase are found in media rich in lignocellulosic waste: in soil, compost, forest litter, in the mass of food waste of plant origin, etc. (Table 1). It is known about the isolation of xylan-decomposing bacteria from marine sediments and from hot geyser springs [35, 36, 37].
Table 1: Lignocellulosic raw material for isolation of xylanase producers
| Raw materials | Identified producers | References |
|---|---|---|
| corn cobs | Aspergillus fumigatus, Kretzschmaria zonata | [38],[45] |
| wheat husk | Aspergillus fumigatus | [40] |
| rice and wheat straw, sugarcane bagasse | Aspergillus, Fusarium, Penicillium, Trichoderma | [39] |
| oil palm waste | Trichoderma, Aspergillus, Rhizopus, Tallaromyces, Penicillium | [41] |
| wood chips | Fusarium compactum | [42] |
| sugar begassa | Lichtheimia ramosa | [44] |
| forest litter | Aspergillus flavus | [46] |
| eucalyptus leaves | Pseudozyma sp. | [48] |
Amat et al. screened xylan-degrading microscopic fungi isolated from degraded corn cobs. A total of 11 strains were isolated, among which the highest xylanase activity (143.0 IU/ml) was found for strain SKF-4, identified as Aspergillus fumigatus. At the same time, the strain had the lowest activity of 2-xylosidase (0.01 IU/ml). The partially purified enzyme from A. fumigatus showed the highest xylanolytic activity at pH 5.0 and 45 °C and was evaluated as promising in terms of XOS production [38]. Analysis of the microflora of various agro-industrial wastes, including rice and wheat straw and sugar cane bagasse, allowed the authors of the study [39] to identify 26 species, representatives of 13 genera, while the enzyme systems of Aspergillus, Fusarium, Penicillium, and Trichoderma showed the highest activity against xylan. Jagtap et al. reported on the isolation of Aspergillus fumigatus R1 strain from wheat husk, a producer of extracellular xylanase with an activity of 152 IU/ml. Cultivation of the strain under optimal conditions on a wheat husk substrate made it possible to obtain the maximum production of XOS, 1% [40].
In Indonesia, xylanase-producing strains from oil palm waste (fruit clusters, leaves, trunk) have been isolated and identified. In total, 32 samples of mold fungi were isolated, exhibiting xylanolytic activity. The isolated microorganisms were identified as belonging to the genera Trichoderma, Aspergillus, Rhizopus, Tallaromyces, and Penicillium. The highest xylanolytic activity, which amounted to 13.35 U/ml, was noted in a strain identified as Talaromyces pinophilus [41]. Isolated strains of microorganisms, twenty of which produced glucanase and xylanase, were obtained from wood chips in India. The Fusarium compactum strain FCGA was identified as the producer of the most active xylanase (9.33 U/ml) [42]. Nour et al. showed a high xylanase activity of the A. terreus strain RGS. Eg-NRC isolated from agro-industrial waste in Egypt by degrading xylan to give XOS. Xylanase with a maximum activity of 245 U/g was obtained by growing the strain on a castor bean substrate [43].
Scientists Alvarez-Zúñiga et al. isolated a zygomycete fungus from sugar bagasse compost in Mexico, identified as Lichtheimia ramosa H71D. It produces the xylanase of 64 kDa, which is named LrXynA, and has an activity of 2.1 U/mL. During the hydrolysis of LrXynA of beech xylan, xylotriose and xylobiose were formed as products; no accumulation of xylose was observed. Based on this, LrXynA was classified as an endoxylanase without β-xylosidase activity [44].
Morales et al. obtained partially purified xylanase when cultivating the pathogenic ascomycete Kretzschmaria zonata of corn cobs. Xylanase showed the highest activity at pH 5.0 and 50 °C, remaining stable at pH values from 4 to 8. Hydrolysis of beech xylan with isolated xylanase yielded xylobiose and xylotriose [45]. The authors of the study [46] isolated a cellulase-free xylanase-producing strain of Aspergillus flavus from forest litter on an inexpensive corn cob substrate with an activity of 1.28 IU/ml. Two strains of bacteria were isolated from the green algae of the Persian Gulf Ulva flexuosa. They produce xylanases active in an alkaline environment, HR05 and HR06, which are most similar to the Bacillus subtilis TN12 strain and the algae strain Shewanella 159418 [47]. Seven strains of microorganisms-producers of xylanolytic enzymes were isolated from eucalyptus leaves. The most active xylanase was found in a yeast strain identified as Pseudozyma sp. Xylanase showed its maximum activity at pH 4.8 and 50 °C. Based on hydrolytic activity, the enzyme was characterized as an endoxylanase similar to those found in the GH 10 family [48]. The variety of strains of microorganisms producing xylan-degrading enzymes indicates the relevance of the search and isolation of new producers from various types of lignocellulosic raw materials, the most accessible of which are agro-industrial waste: corn cobs, rice, wheat, oil palm processing waste [38, 39, 41, 42].
The prebiotic effect of XOS lies in their selective stimulation of growth and modulation of the beneficial microflora of the colon, an increase in the number of bifidobacteria due to immunostimulation, as well as the regulation of insulin secretion by the pancreas. Like other prebiotics, XOS is fermented to short-chain fatty acids in the lower gastrointestinal tract. The resulting compounds contribute to a decrease in pH and may be associated with the prevention of intestinal infection, the suppression of the development of colon cancer, and the overall improvement of intestinal health [1, 5]. XOS are used predominantly by bifidobacteria Bifidobacterium spp, especially Bifidobacterium adolescentis and Bifidobacterium longum, and they are 20 times better utilized by them than fructooligosaccharides. At the same time, XOS does not contribute to the increase in the number of bacteria of the genus Lactobacillus, the excess content of which is harmful to obese people. The ability of XOS to suppress the development of negative microflora and have a beneficial effect in irritable bowel syndrome was also noted [49, 50].
Studies have shown that XOS in the human body exhibits immunomodulatory, anti-inflammatory, antioxidant, antitumor, and antimicrobial activity. It has been shown in vitro that XOS has an immunomodulatory effect by regulating the expression of anti-inflammatory mediators. Feeding XOS to experimental animals resulted in a significant reduction in systemic inflammation [6]. The anti-inflammatory effect of XOS has a beneficial effect on the course of the so-called metabolic syndrome: XOS helps control body weight and regulate glucose and lipid homeostasis and insulin sensitivity of tissues [5]. In rodent model studies, AXOS has been found to be useful in counteracting the development of fat mass and hyperinsulinemia by enhancing intestinal barrier function [51].
The use of XOS for eight weeks contributed to the normalization of blood glucose levels and improved lipid profile. It was noted that the level of antioxidant enzymes superoxide dismutase and glutathione peroxidase increased in the subjects' organisms [52]. XOS is able to change the structure and physicochemical properties of the membrane bilayer, causing changes in the sphingomyelin/cholesterol ratio in rat liver plasma membranes, thereby having a beneficial effect on glucose and lipid metabolism [53].
The anticarcinogenic effect of XOS against colon cancer was manifested in a significant increase in the population of bifidobacteria and a decrease in the frequency and multiplicity of benign precancerous lesions in the colon [54]. XOS has also been found to have an antitumor effect on human breast cancer. XOS dose-dependently reduced the number of viable cancer cells and induced their apoptosis [55].
XOS exhibits antimicrobial activity against both Gram-negative and Gram-positive unwanted bacteria. XOS supplementation markedly lowered the pH level of the cecum and not only increased the population of bifidobacteria but also decreased the number of E. coli. Inhibition of the growth of pathogenic microorganisms is also facilitated by the formation of lactic and acetic acids when using XOS [6, 56].
Many studies have established the antioxidant potential of XOS derived from corn cobs, sunflower, wheat stalks, sugar cane begass, wheat, rice, and corn bran. Exhibiting antioxidant properties, XOS is able to absorb free radicals, which is explained by the efficient release of phenolic compounds (derivatives of hydroxycinnamic acid ester-bound, such as ferulic acid and syringic acid residues) and the transfer of hydrogen atoms from phenolic compounds to free radicals [5]. Antioxidant activity for different XOS is not the same and depends on the type of substitution. Thus, the antioxidant activity of UXOS is higher than that of AXOS [57, 58]. The highest antioxidant activity was found for XOS with ferulic acid substituents [59]. Studies have shown that feeding XOS from corn cobs improves clinical conditions associated with diabetes, improves nephron function, restores antioxidant enzyme activity, and significantly reduces rodent mortality [60]. Wheat bran XOS has been found to be effective in protecting humans from oxidative stress on a high-fat diet [61].
A wide range of pharmacological activity of XOS, their prebiotic, immunomodulatory, anti-inflammatory, antioxidant, antimicrobial, and anticancer properties, determine the great potential for the use of these components in the composition of dietary supplements for human nutrition. The development of supplements in the form of XOS, as well as complex supplements containing other prebiotic components, is one of the priority areas for the development of the healthy nutrition industry [1, 5, 6, 60, 61]. The selection of lignocellulosic raw materials and the identification and analysis of new strains sources of xylanolytic enzymes are important stages in the development of cost-effective technologies for obtaining useful nutritional components.
XOS is a new generation of functional components with great potential for use in feed, nutrition, and medical purposes due to their prebiotic, antioxidant, immunomodulatory, and antitumor properties. The main sources for obtaining XOS are xylans of lignocellulosic raw materials, which can be split to XOS by chemical, enzymatic hydrolysis, or physical treatment. Enzymatic hydrolysis of xylan with xylanolytic enzymes makes it possible to obtain products of high purity and the required physiological action. The ubiquity of microorganisms that utilize xylans provides a means to identify the producers of the most active xylanases among microscopic fungi, bacteria, and actinomycetes from the soil, various agricultural plants, and waste products from their processing.
The article was financially supported by the RSF grant, agreement No. 23-26-00091.