2025 Volume 13 Issue 1 Pages 66-80
2025 Volume 13 Issue 1 Pages 66-80
The soil neutralization process is usually accompanied by various kinds of changes including soil physicochemical properties and microbial diversity and composition. This is especially significant for tea plants, which thrive in acidic soils. The neutralization process, which involves reducing soil acidity through organic management, has been continuously occurring in tea plantations to mitigate the negative effects of soil acidification. However, there is limited information on how soil neutralization affects microbial communities, particularly in terms of nutrient cycling, decomposition of organic matter, and plant growth promotion. Therefore, we summarized the current study focusing on soil neutralization in tea plantations. Firstly, we identified some effective and sustainable management for soil neutralization that balance pH modification with enhancing soil health and investigated the dynamic changes in soil microbial communities in tea plantations. Besides, we highlighted the role of beneficial microbes linked to specific attributes of tea quality. Our study provides insights, from improving soil health and microbial communities to enhancing tea quality during soil neutralization, ultimately supporting the production of high-quality tea in sustainable management.
Tea (Camellia sinensis L) is one of the most important cash crops throughout the world and plays a significant role in supporting the economies of major tea-producing countries including China, India, and Kenya (Fig. 1a). Over the past decades, the global tea industry has seen rapid growth, with a rising number of tea production (increasing 19,206,317 t) and tea harvested area (increasing 3,004,442 ha) from 1992 to 2022 (Fig. 1b). However, the production and harvested area in tea regions of Japan has been gradually declining year by year (Fig. 1c). Among the reasons, it is not only related to the aging workforce, declining consumption, and climate change but also related to soil degradation [1, 2]. Soil acidification is exactly a serious aspect of soil degradation worldwide and has been reported in a variety of crop ecosystems [3, 4, 5]. Generally, tea plants prefer acidic soils, unlike other crops, and tend to apply heavy chemical fertilizer (especially nitrogen fertilizer) to achieve high tea yields and quality content, further causing more severe soil acidification and environmental pollution. Yang et al. [6] confirmed that soil acidification naturally occurred in tea plantations with an annual rate of 0.07. Yan et al. [7] showed that the soil pH maximally decreased by 1.43 in tea plantations with chemical fertilization, but no significant acidification was observed in tea plantations with organic fertilization. So, organic management is an effective measure for neutralizing acidified soil in tea plantations.

Tea leaves are rich in specialized metabolites including catechins, caffeine, theanine, and aroma compounds, which contribute to the rich taste, flavor, and health benefits of tea beverages [8]. Generally, tea cultivars characterized by rich metabolite concentrations determine the final quality and economic value of the tea crop. For example, “Longjing43” of China, “Yabukita” of Japan, and “Assam” and “Darjeeling” of India. However, the abundance of these characteristic compounds is not only dependent on genetic background, the time of harvest, and the manufacturing process, but also is affected by the surrounding soil environment, including soil nutrient composition and the soil microbial community [9]. Soil microbiomes, especially the rhizosphere zone, can create more opportunities to promote nutrient mineralization and availability, and they play important roles in plant health and development [10]. Rhizosphere microbial communities showed a greater response than soil properties to tea plants and were sensitive to the changes in root secretion, fertilizer management, and abiotic stress in tea soils [11,12]. Therefore, exploring soil neutralization mainly focuses on changes in soil microbial communities and the complex soil-microbe-plant interactions are further indicated in our study.
In this review, we have summarized the effects posed by soil acidification, the measures taken to neutralize tea soils, and the changes in the composition and function of soil microbiomes during soil neutralization. We have also described the relationships between soil microbes and nutrient uptake by tea plants. Gaining a thorough understanding of microbial properties and changes during soil neutralization could provide new insights to assist in improving management practices and tea production. Future studies into developing and culturing tea-associated microbial strains will be required to improve soil environments and promote sustainable tea plantations.
Soil acidification is a major threat to agricultural ecosystems worldwide and occurs in many tea-growing countries. In the 1960s, the range of soil pH in tea plantation soils was between 5 and 6 worldwide, and soil pH dropped to 4–5 until the 1980s, especially when Japan experienced particularly severe acidification [13, 14]. As shown in the National Greenhouse Gas Inventory Report of Japan of 2023, the planted area of tea plants decreased year by year, but the amount of synthetic fertilizer application per area increased gradually, and the emission factor of tea was significantly higher than that of other crops. In China, only 43.9% of the tea soils had a pH in the interval of 4.5–5.5, while 46.0% of the soil samples had a pH < 4.5. Compared with other crop systems, the highest rate of acidification was observed in soil under tea cultivation [7]. Despite differences in planting conditions across different countries, the common feature is the application of excessive N fertilizers to achieve high levels of free amino acids in tea leaves, thereby producing high-quality tea products. However, the application of N fertilizer directly accelerated soil acidification by producing hydrogen ions (H+) during the nitrification process, leading to a decrease in alkaline cations [15]. Continuous nitrogen fertilizer application also decreased soil microbial diversity, weakened community function and stability, and further affected tea yield and quality [16, 17]. In addition to the above reasons, soil acidification was attributed to the tea plant’s own physiology. Large amounts of Al were accumulated in tea leaves and the biogeochemical cycle of Al in tea and soil was another factor that led to soil acidification in tea plantations [18]. Xue et al. [19] also investigated three tea plantation systems of different ages, which indicates that the pH of tea soils gradually decreases with age increasing. Therefore, the fundamental approach to alleviating soil acidification in tea plantations is to apply organic management and neutralize acidic soils.
Traditionally, biochar and lime are commonly used soil amendments that have been confirmed to mitigate soil acidification for many years in various crops. Liang et al. [20] showed that quicklime had a significant impact on the pH, cation exchange capacity, NO3–-N, NH4+-N, K, and P of strongly acidic soils of tobacco plants. Malik et al. [21] revealed that soil pH was increased by 64%, and the contents of exchangeable H+ and Al3+ were reduced in strongly acidic soils after the application of different kinds of biochar and lime, which further increased the wheat biomass and yield. In tea soils, the addition of quicklime and biochar was considered a win-win strategy to alleviate acidification and inhibit nitrification and N2O emissions [22, 23]. But other studies showed that biochar application seemed to be a more suggested amendment to ameliorate acidic tea soils than lime application, which could stimulate tea growth by improving leaf area, supplying P, K, and Mg, and reducing the heavy metals Mn and Cu [24, 25]. At present, Han et al. [25] also indicated that manure application rather than biochar increased acidic soil respiration in the tea plantation. Organic fertilizers are usually complex compounds that add numerous organic matter and micronutrients, which are different from pure and simple chemicals. It usually is often derived from animal manure, plant wastes, and sewage sludge [26]. Whalen et al. [27] early stated that cow manure amendments significantly resulted in an immediate increase in the pH of acid soil and supplied considerable quantities of plant-available nutrients in the short term. These effects have been extensively validated in many crops including maize, apple, and tomato systems [28, 29, 30].
Over 20 years ago, organic fertilization has been applied to mitigate soil degradation and acidification in tea-growing countries. Compared to the conventional tea field, the organic farming system could result in significantly higher soil pH, leaf length, leaf width as well as major polyphenol content and yield in tea shoots [31]. Different organic patterns could affect soil ammonium nitrogen and amino acid nitrogen, and further affect the accumulation of theanine in tea leaves [32]. Tea farmers started to choose more and more varieties of organic fertilizers and combinations to neutralize acidic tea soils such as manure (cow, sheep, pig, chicken manure), intercropping green manure, municipal solid waste compost, plant wastes (soybean cake, rapeseed cake). Ji et al. [33] conducted a long-term field experiment with organic fertilization with different substitution ratios, which indicated that increasing the organic substitution ratio could increase soil carbon and nutrients and 25% N substituted with organic fertilizer produced a higher tea yield. On the other hand, organic fertilization not only improves the soil environment but also affects the aroma, taste, and quality components of tea leaves. Sun et al. [34] investigated that the application of cow manure significantly enriched metabolic pathways related to amino acids, sugars, and fatty acids in tea shoots, and showed higher levels of aroma when used to make the tea. Huang et al. [35] further showed the application of organic fertilizer combined with tea-specific fertilizer significantly improved the concentration of green tea aroma compounds, such as D-limonene, cis-jasmone, nonanal, and linalool. Other studies demonstrated that organic tea contained higher contents of polyphenols, some catechins such as epicatechin gallate (ECG), epigallocatechin gallate (EGCG), epicatechin (EC), epigallocatechin (EGC), and the amino acids inclusive of γ-aminobutyric acid (GABA) and proline, which were associated with the plant defensive mechanisms and the plant protection against environmental stress [36]. Recently, Raza et al. [37] indicated that organic fertilization upregulated the expression of most structural genes involved in the flavonoid biosynthesis pathway, and the high expression of DFR2, ANS3, and ANR2 promoted the accumulation of total catechins. Accordingly, in tea plantations with already acidified soils, long-term organic fertilization could reduce soil acidification, further conducive to tea growth, quality, and yield reaching the maximum levels [38].
The evaluation of soil condition is a multiple-index system including physical, chemical, and biological indicators. Previous studies only focused on nitrogen, phosphorus, and potassium as the physicochemical index to reflect soil nutrient status, and do not directly link the microbial information with soil properties of the soil dynamic process [39]. The biological indicators, such as microbial composition, diversity, and function, may best predict shifts in soil complete status [40]. At present, the integration of advanced high-throughput sequencing techniques has elucidated more detailed characterization and functional potential of microbial communities, which can cope with different soil environments.
Under chemical fertilization, Geisseler and Scow [41] analyzed the responses of soil microorganisms to mineral fertilizer from 64 long-term trials from around the world and summarized that long-term repeated mineral fertilization could alter the microbial community composition and specific microbial groups even with small changes in pH value. For example, the relative abundance of denitrifying bacteria belonging to Pseudomonas decreased, while those of Nitrosospira and Azoarcus increased in soils with long-term nitrogen fertilization, and Actinobacteria was recognized as an indicator of a decreased soil pH caused by nitrogen fertilization [42, 43]. Under organic fertilization, Ling et al. [44] collected soil samples from long-term field experiments initiated in 1978 and suggested that long-term organic fertilization enhanced bacterial connectivity and showed stronger functional potentials and more interactions within the soil community relative to long-term chemical fertilization. Organic fertilizer itself contains a large amount of organic matter and microbial resources, and it could expand and stimulate soil native microbial communities including biomass, gene abundances, and respiration after application [45]. However, soil bacteria and fungi responded differently in the face of changes in soil environments. Lauber et al. [46] showed that soil pH was the best predictor of bacterial community composition while some soil nutrients were more closely associated with soil fungal communities. Rousk et al. [47] also showed that bacterial growth was more sensitive to pH changes than fungi, indicating that fungi could maintain more functionality under a broader range of soil pH. An investigation of global topsoil samples (189 sites, 7,560 subsamples) further indicated that soil pH showed the strongest response to the diversity and composition of soil bacterial communities [48].
4.1 Soil bacterial communities during soil neutralizationAcidic soils, common in tea-growing regions, typically harbor different microbial communities compared to neutral or alkaline soils. Zheng et al. [49] found soil pH during tea planting was the most important factor affecting the soil microbial community, while soil bacteria were different from fungi. Besides, the soil neutralization process in tea plantations involved significant changes in soil pH. Therefore, the composition and changes of microbial communities in responding to soil neutralization in tea plantations should be comprehensively summarized. Firstly, Chen et al. [50] summarized bacterial communities of different niches of tea plants and indicated that the proportion and species differed from the rhizosphere to the bulk zone due to the rhizosphere effect. Specifically, Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteriota, and Firmicutes dominated in tea bulk soils, and Proteobacteria, Firmicutes, and Acidobacteria enriched in tea rhizosphere. In our study, the dominant phyla in soil with different organic fertilizations were focused on exploring changes in the soil neutralization process (Table 1). Although the soil environment had a relatively stable phylum system, the proportion of major bacterial phyla in soil (for example, Proteobacteria, Acidobacteria, and Actinobacteria) in the soil bacteria ecosystem was unpredictable. Proteobacteria, as the largest phylum of bacteria, cover a wide range of physiological metabolic types due to their rich genetic diversity. Smit et al. [68] indicated that α- and γ-Proteobacteria showed potentially high growth rates in the soil environment with high available nutrients. Meanwhile, Acidobacteria was easy to survive in soils with low-nutrient and recalcitrant substrates and have a higher capability to compete for substrates. Torsvik and Øvreås [69] further showed that the ratio between the number of Proteobacteria and Acidobacteria (P/A ratio) might be indicative of the soil trophic status. After organic fertilization, the abundance of Proteobacteria significantly increased in tea soils with the increase in soil pH and organic matter [32, 33, 56, 58, 59]. Besides, the P/A ratio in tea soils with organic fertilization was higher than that in soils with urea, also indicating that the input of organic matter can adjust the P/A value to show the soil nutrient status during soil neutralization [55]. Actinobacteria contribute to global carbon cycling through the decomposition of soil organic and increase plant productivity in terrestrial ecosystems [70, 71]. It exactly is the possible reason for the higher abundance of Actinobacteria in soil under organic fertilization in tea plantations. They can produce more extracellular enzymes including cellulases, proteases, and amylases, and convert complex organic matter into simple small molecules that can be more easily absorbed and utilized by tea plants during soil neutralization. Bacteroidota is an important microbial community not only in soil but also in the organic compost process. They were predominant with high abundance in manure composting and played an important role in organic matter degradation and nutrient conversion [72, 73, 74]. Besides, Yang et al. [29] reported that the bacterial phyla Rokubacteria, Gemmatimonadetes, and Verrucomicrobia were greatly enriched in tea soil with organic fertilization, which indicated rare taxa, rather than the dominant taxa, were key variables for predicting soil condition. In the process of gradual neutralization of soil acidity, soil bacterial communities (dominant and rare taxa) were more interactive and multifunctionality, thereby alleviating the stress caused by soil acidification and improving soil quality in tea plantations.
4.2 Soil fungal communities during soil neutralizationOrganic fertilization in tea plantations strongly modified the composition of fungal communities but not the richness and diversity in acid tea soils [28]. Yang et al. [75] reported that long-term nitrogen fertilization in the tea plantation significantly reduced soil fungal community diversity and altered the composition stricture, and soil NO3–-N and pH were identified as the negative factors. But in neutral tea soils, fungi have developed a new aspect of heterotrophic nitrification and a significant contribution of nitrification potential to improve tea quality [76]. Chen et al. [50] summarized that Ascomycota, Zygomycota, and Basidiomycota were common dominant phyla in tea rhizosphere bulk soils. Each of these fungi played different roles and functions in soil nutrient cycling and organic matter decomposition. In our study, the dominant phyla in soil with different organic fertilizations were focused on exploring changes in the soil neutralization process and showed the dominant fungi were Ascomycota, Basidiomycota, Mortierellomycota and Zygomycota (Table 1). Ascomycota and Basidiomycota were recognized as the most abundant phyla in the global soil survey. These abundances were positively correlated with soil pH, organic carbon, and soil moisture [66]. This correlation exactly corresponded to the changes in soil neutralization, suggesting that these fungi thrive in improved soil conditions facilitated by organic fertilization. Manici et al. [77] recently showed Ascomycota had higher adaptability to metabolize different crop residues and litters, and Basidiomycetes could degrade plant polysaccharides and decompose lignin. The diversity of compounds in plant litter resulted in corresponding changes in fungal community composition. Organic fertilizers could bring in carbon inputs, and the amount of carbon inputs not only increased soil fungal biomass but also selected for specific fungal taxa, thus also altering the composition of fungal community [78]. Therefore, fungal communities mainly responded to the amount of organic fertilizer provided and were easy to show more dynamic characteristics with the time after organic fertilization.
Table 1: The dominant soil microbial communities after organic fertilization
| Organic fertilization | Dominant microbial communities | Enrich soil microbial phyla after organic fertilization | References |
|---|---|---|---|
| Bacterial communities | |||
| Chicken manure | Acidobacteria, Proteobacteria, Actinobacteria, Chloroflexi, Verrucomicrobia, Bacteroidetes |
(Phylum) Proteobacteria, Bacteroidetes, Planctomycetes, Gemmatimonadetes, Verrucomicrobia |
[33] |
|
Commercial organic fertilizer; Cow manure: Pig manure: Insect |
Proteobacteria, Acidobacteria, Chloroflexi, Actinobacteria, Firmicutes, Thaumarchaeota |
(Phylum) Verrucomicrobia, Bacteroidete, Planctomycetes |
[51] |
|
Rape cake; Sheep dung |
Actinobacteria, Chloroflexi, Proteobacteria, Acidobacteria, Gemmatimonadetes, Cyanobacteria | (Order) Burkholderiales, Myxococcales, Streptomycetales, Nitrospirales, Ktedonobacterales, Acidobacteriales, Gemmatimonadale, Solibacterales | [52] |
|
Commercial organic fertilizer; Pig manure; Cow manure |
Proteobacteria, Acidobacteria, Chloroflexi, Firmicutes, Chlamydiae, Verrucomicrobia, Bacteroidetes, Planctomycetes |
(Phylum) Acidobacteria, Proteobacteria, Bacteroidetes, Verrucomicrobia, Planctomycetes, Nitrospirae |
[53] |
|
Soybeans; Green manure; Goat manure |
Proteobacteria, Acidobacteria, Chloroflexi, Actinobacteria | (Family) Rhizobiaceae, Bradyrhizobiaceae | [54] |
| Cow manure | Proteobacteria, Bacteroidetes, Acidobacteria, Actinobacteria | (Phylum) Bacteroidetes, Proteobacteria (Genus) Pedobacter, Flavobacterium |
[55] |
|
Commercial organic fertilizer; Rapeseed cake |
Proteobacteria, Actinobacteria, Acidobacteria, Chloroflexi, Firmictues |
(Phylum) Chloroflexi, Betaproteobacteria, Gaiellales, Nitrospirae, Alphaprobacteria, Solirubrobacterales (Genus) Conexibacteraceae, Geodermatophilaceae, Thermomonosporaceae |
[56] |
| Rapeseed cake; Green manure |
Acidobacteria, Proteobacteria, Actinobacteria |
(Phylum) Acidobacteria, Proteobacteria, Actinobacteria (Genus) Acidibacter, Acidothermus |
[57] |
| Biochar | Proteobacteria, Actinobacteria, Chloroflexi, Acidobacteria |
(Phylum) Gemmatimonadetes,
Firmicutes, Proteobacteria, Bacteroidetes, TM6_Dependentiae (Genus) Candidatus_Solibacter, Gemmatimonas, Singulisphaera, Aquicella, Rhodoplanes, Pseudarthrobacter, Sorangium |
[58] |
| Bio-organic | Betaproteobacteria, Acidobacteria, Alphaproteobacteria, Gammaproteobacteria |
(Phylum) Alphaproteobacter, Acidobacteria, Deltaproteobacteria, Planctomycetes |
[59] |
| Commercial organic fertilizer | Proteobacteria, Acidobacteria, Chloroflexi, Actinobacteria, Firmictues | (Phylum) Rokubacteria, Gemmatimonadetes, Verrucomicrobia, | [60] |
| Fermented soybean | Proteobacteria, Bacteroidota, Firmicutes, Acidobacteriota, Actinobacteriota |
(Genus) Glutamicibacter, Streptomyces |
[61] |
| Biochar; Cow manure | Acidbacteria, Actinobacteria, Bacteroidetes, Chloroflexi, Proteobacteria, | (Phylum) Gemmatimonadetes | [62] |
|
Biochar; Tea tree litter |
Proteobacteria, Acidobacteria, Actinobacteria, Candidate, WPS−2, Chloroflexi | - | [63] |
|
Soybean meal; Furfural residue; Straw |
Proteobacteria, Acidobacteria, Actinobacteria | (Phylum) Proteobacteria, (Class)Gammaproteobacteria | [64] |
| Leguminous green manure | Proteobacteria, Acidobacteria, Actinobacteria, Gemmatimonadetes, Bacteroidetes |
(Genus) Mycobacterium, Burkholderia, Paraburkholderia |
[32] |
|
Poultry manure; Straw |
Proteobacteria, Acidobacteria, Actinobacteria, Bacteroidetes, Chloroflexi, | - | [65] |
| Fungal communities | |||
| Chicken manure; Legume straw | Ascomycota, Basidiomycota, Zygomycota, Glomeromycota, Chytridiomycota | (Genus) Fusarium, unclassified_Microascaceae, unclassified_Ascomycota | [66] |
| Pig manure | Ascomycota, Zygomycota, Basidiomycota | (Phylum) Basidiomycota | [67] |
| Biochar | Ascomycota, Basidiomycota, Mortierellomycota, Glomeromycota, Mucoromycota |
(Phylum) Mortierellomycota, Mucoromycota, Rozellomycota (Genus) Aspergillus, Chaetomium, Clitopilus, Chaetosphaeria, Mortierella |
[58] |
| Commercial organic fertilizer |
Ascomycota, Basidiomycota, Mortierellomycota, Mucoromycota, Rozellomycota |
(Phylum) Mortierellomycota | [60] |
| Fermented soybean | Ascomycota, Mortierellomycota, Rozellomycota, Glomeromycot, Mucoromycota | (Genus) Candida Actinomucor | [61] |
|
Biochar; Tea tree litter |
Ascomycota, Basidiomycota, Chytridiomycota, Mortierellomycota |
(Phylum) Ascomycota (Genus) Talaromyces |
[63] |
|
Soybean meal; Furfural residue; Straw |
Ascomycota, Basidiomycota | (Class) Tremellomycetes | [64] |
Plants as an integrated system of multiple interacting components are colonized by a rich diversity of microbes, of which some members are known to promote plant growth and health [79]. Meeting soil improvement and sustainability amidst the soil neutralization process requires integrating the reliability, resource use, and environmental impacts of beneficial microbiomes, which to further explore plant-microbiome interactions in the context of organic agriculture. Zhao et al. [80] highlighted that chemical fertilization reduced ammonia-oxidizing bacteria and some predatory bacteria in acidic soils and altered entire microbiome functioning. Jing et al. [81] also showed agricultural intensification resulted in soil microbiomes being weakened to their capacity for multiple functions and other fertilizer management could create positive aboveground and belowground relationships.
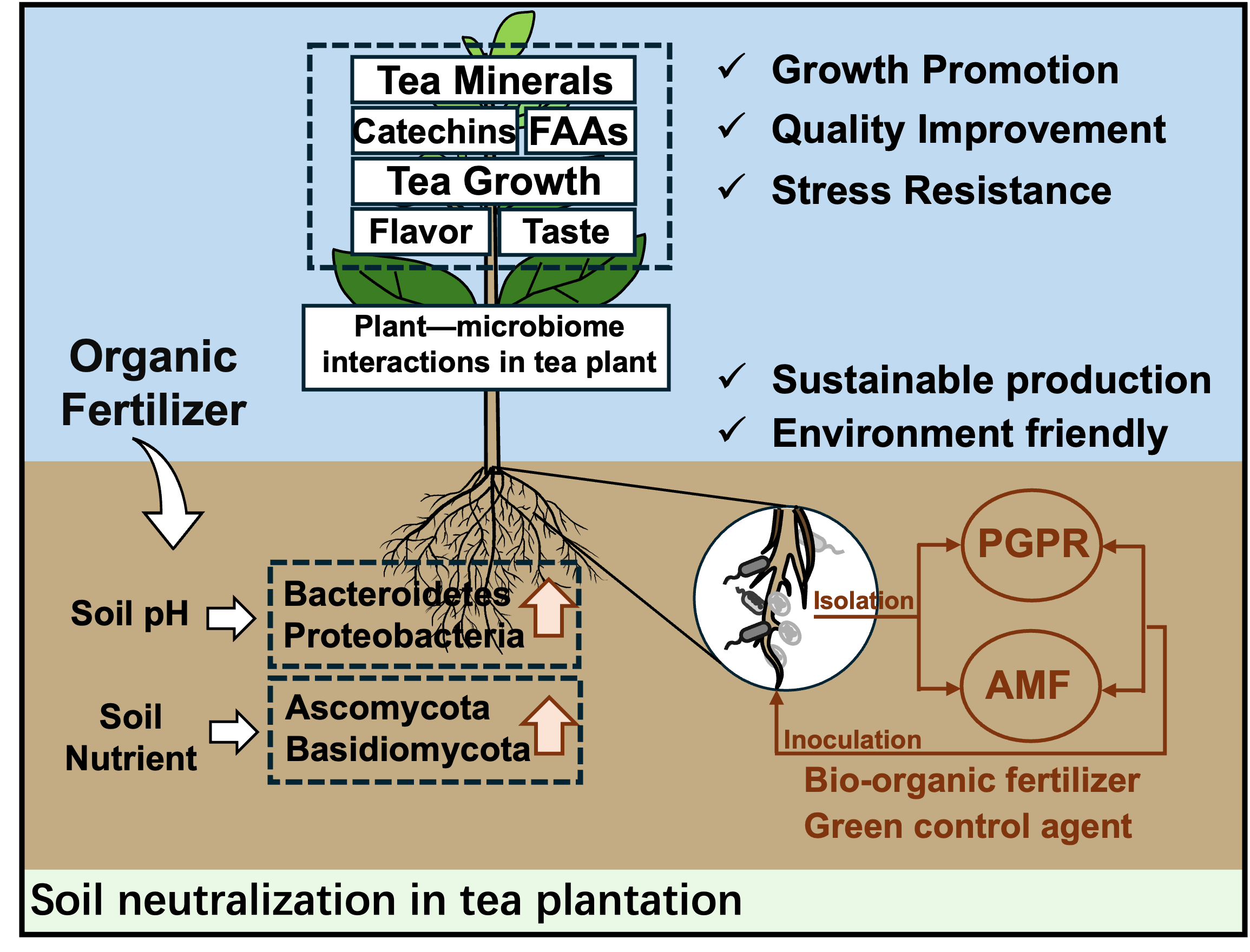
5.1 The functions of plant growth promoting bacteriaThe development of plant growth promoting bacteria (PGPB) is recognized as a green technology that can reduce the application of chemical fertilizers thereby improving soil health. Recently, some researchers have tried to select PGPBs generated from the rhizosphere zone in tea soils under organic cultivation. Bacillus sp. was the predominant bacteria of the phylum Firmicutes with plant growth promoting activity. Bacillus vallismortis TR01K and Bacillus subtilis BRAM_G3, as strains of potent plant growth-promoting rhizobacteria (PGPR) isolated from tea soils, showed important potential to form a significant biofilm and produce plant growth hormones (IAA, GA3, Cytokinin) [82]. These two strains were applied again to indicate that they had abilities to increase tea productivity while reducing soil heavy metal contents under organic farming. Other Bacillus strains (Paenibacillus sp. YN15, Bacillus sp. BIHB 344, and Bacillus sp. DTG11) were also indicated to have tea growth-promoting features such as nitrogen fixation, phosphate solubilization and pest controlling [9]. Bhattacharyya et al. [83] selected 30 potential rhizobacterial isolates from different tea fields, which were identified as belonging to the genera Pseudomonas, Lysinibacillus, Micrococcus, Leifsonia, Exiguobacterium, and Arthrobacter, finally resulted in growth promotion based on in-planta evaluation in maize and rice crops. Besides, Brevibacterium sediminis A6 (family Actinobacteria) isolated from the tea rhizosphere has the potential for plant growth promotion and further produced biosurfactant by utilizing dextrose as the carbon source [84]. Theanine, as a pivotal compound affecting tea quality, was strongly associated with the absorption and utilization of ammonium. Zhou et al. [85] indicated that Bacillus could produce organic acids to increase soil available potassium content, which further activated recombinant CsTSI activity (theanine biosynthesis enzyme) and increased ethylamine content, resulting in theanine synthesis in tea roots. Xin et al. [86] utilized a synthetic microbial community (most from phylum Proteobacteria) from tea roots of high-theanine cultivars, which resulted in increasing in the theanine content of tea plants and imparted tolerance to nitrogen deficiency in Arabidopsis. Usually, the primary purpose of applying nitrogen fertilizers is to improve the level of theanine, while it leads to a large amount of residual fertilizer and accelerates soil acidification. Based on this background, these PGPRs will provide an environmentally friendly strategy for developing innovative functional bio-organic fertilizers to enhance tea quality.
5.2 The functions of arbuscular mycorrhizal fungusThe arbuscular mycorrhizal fungus (AMF) supported that tea plants healthily grew in acid soils and are usually recognized as the resources of bio-organic fertilizers. They could form complex consortia through mutualism, commensalism, and parasitism with plant roots. In mycorrhizal associations, AMF positively benefited plants by improving the absorption of essential micronutrients such as zinc, copper, and iron, increasing water uptake, and resisting environmental stresses. Singh et al. [87] early observed that Acaulospora, Gigaspora, Glomus, and Scutellospora existed in natural tea soils. They also showed a significant increase in the growth and quality parameters (root and shoot length, dry weight, total polyphenols, and caffeine contents) of tea plants through AMF inoculations [88]. Sharma et al. [89] compared the effectiveness of AMF species (Acaulospora scrobiculata, Glomus macrocarpum, Rhizophagus intraradices) on tea growth and indicated that AMF inoculation, regardless of single or mixed AMF consortium, had a significant positive impact on the seedling growth of tea. Besides, the contents of N, P, K, Ca, Mg, Zn, and Mn in tea leaves were significantly higher in AMF-inoculated plants (Claroideoglomus etunicatum, Diversispora spurca, D. versiformis and a mixture of the three AMF species) than in non-AMF-inoculated plants [90]. Among soil factors (pH, N, P, K and organic matter), the root AMF rate of tea plants was significantly influenced by soil organic matter [91]. Although AMF has shown significant production benefits in symbiosis with plants under laboratory conditions, their performance in natural environments may be affected by various environmental factors. Therefore, implementing targeted AMF inoculation techniques in the field and combining them with organic fertilization can maximize these benefits, leading to higher-quality tea production. In tea plantations, Kumla et al. [92] isolated and identified 35 yeast strains (belonging to the phylum Ascomycota and Basidiomycota, the genera Galactomyces and Wickerhamomyces, and potential new species) from tea soils in Assam and showed multifarious plant growth promoting traits. After applying organic fertilizer, yeast can decompose complex organic matter from manure and release essential nutrient elements for plant utilization. Sometimes, yeast suspensions usually have been used the foliar fertilizers and compost tea, but it should be noted that some yeast strains were potential threats to plants and humans. Therefore, toxicity assays in the laboratory are needed before these yeast strains are applied to fully understand the profile.
Our study first illustrated how the application of organic fertilizers in tea plantations can lead to improved soil health by altering microbial communities, which in turn enhances tea plant growth and quality, and focused on beneficial microbes, such as PGPR and AMF, to develop new bio-organic fertilizers in sustainable and environmentally friendly tea production (Fig. 2). However, there remain some gaps in our understanding. Addressing these gaps through targeted research can lead to optimized soil management practices that enhance both soil health and tea production.
1) Most studies on soil neutralization focused on short-term effects, with a limited understanding of the long-term impacts on tea plants. Even related to the long-term application, future studies need to assess how continuous soil neutralization affects tea plant growth, yield, and quality contents by monitoring changes in soil properties and microbial communities over time.
2) The detailed knowledge about how soil neutralization alters the composition and function of microbial communities, particularly beneficial microbes such as PGPRs and AMF is unclear in tea soils. Advanced molecular techniques like metagenomics should be used to characterize the shifts in microbial communities and their functional roles.
3) Most research recently only showed the effects of soil neutralization on tea growth and developments. Future analyses will strengthen positive soil-microbe-plant interactions, namely relationships among soil properties, soil microbial communities and key secondary metabolites in tea leaves. Optimizing nutrient management strategies by organic fertilization should be conducted to determine the most effective formulations for improving the concentration of amino acids, polyphenols, catechins, and other bioactive compounds.