2020 年 37 巻 3 号 p. 95-101
2020 年 37 巻 3 号 p. 95-101
Objective: In gastrointestinal endoscopy, the bleeding volume does not increase, even if a biopsy is performed under continuous warfarin administration. However, the safety when anticoagulant therapy is continued during flexible bronchoscopy has not been reported. We measured the actual blood loss in patients under continuous warfarin administration. Methods: This study was designed as a single-center, double-arm, prospective clinical trial. The bleeding volume during the procedure was calculated from aspirated secretions with a portable hemoglobin-measuring device. Results: Twenty-three subjects in the control group and 5 in the warfarin group were enrolled over a 60-month period. Overall, the blood loss in the warfarin group was comparable to that in the control group (median 0.2 vs. 0.8 ml). No subject experienced severe blood loss. All patients in the warfarin group used a guide sheath. Conclusions: Although limited to peripheral lesions with a guide sheath, we found no severe bleeding in the warfarin group.
With the aging of society and advances in preventive medicine, the number of patients taking antithrombotic agents has been increasing in recent years. About 15% of patients undergoing gastrointestinal endoscopy are taking antithrombotic therapy at the time of their examination [ 1]. In the guidelines for the examination and treatment in each field, it is emphasized that attention must be paid to both the risk of bleeding due to antithrombotic drugs and the risk of thromboembolism caused by their withdrawal.
In the field of bronchoscopy, prospective cohort studies with antiplatelet drugs (aspirin) have been reported, and no increase in bleeding was observed even under continuous aspirin administration [ 2]. However, another antiplatelet drug (clopidogrel) was found to be associated with an increase in the amount of bleeding, so research was stopped at an early stage [ 3]. Regarding the anticoagulant warfarin, no correlation was noted between the prothrombin time-international standard ratio (PT-INR) and bleeding volume in mini-pigs (e.g. PT-INR>10) [ 4].
Although data suggesting the possibility that neither antiplatelet drugs nor anticoagulants might affect the risk of bleeding has been obtained, data on such patients are either small or absent, so it is considered ideal to conduct biopsies after a period of withdrawal of the drug, if possible [ 5].
However, medicine cannot be withdrawn in all cases. For example, if a patient has a history of valvular surgery, warfarin cannot be switched for a direct oral anticoagulant (DOAC). Low-molecular-weight heparin replacement is used in such cases. However, recent reports that analyzed the efficacy and safety of bridging therapies during anticoagulation interruptions, replacements of warfarin to short acting anticoagulants, during the invasive procedure revealed the rather higher risk of bleeding with such bridging therapies when compared with nonbridging patients [ 6- 8].
Advances in bronchoscopy have been achieved, with approaches to peripheral pulmonary lesions now possible using devices such as guide sheaths and endobronchial ultrasonography, suggesting that the safety of bronchoscopy is improving [ 9- 11]. In a prospective study conducted in recent years, the amount of bleeding in 90% of cases was < 5 ml, and severe bleeding was not reported [ 12].
In the present study, we hypothesized that there would be no change in the bleeding volume even if warfarin was continued. Antiplatelet drugs reported to increase bleeding in patients receiving a bronchoscopic biopsy were excluded from the evaluation.
Study Population
Patients referred for bronchoscopy to the Division of Medical Oncology and Respiratory Medicine, Shimane University Hospital during the period from May 2013 to April 2018 were potential candidates for this study. All patients over 18 years old who gave their informed consent were enrolled when the criteria lists were satisfied ( Table 1). Shimane University hospital is a 600-bed academic facility in Izumo City, Japan. The study was approved by the Shimane University Research Ethics committee (approval number: 2319, approval date: May 29, 2013) and was performed in accordance with the principles of the 1964 Declaration of Helsinki.
A clinical history, including screening questions for bleeding tendencies, was taken. Blood tests were done within six hours before bronchoscopy. We measured the full blood count, partial thromboplastin time, PT-INR, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin blood (T-bil) and serum creatinine (Cr). Previous reports suggested that a platelet count (PLT) of at least 50 × 103/μl was suitable for bronchoscopy [ 13, 14]. We decided to set the lowest acceptable PLT at 50 × 103/µl.
| Inclusion criteria |
| 1. Bronchoscopy patients |
| 2. Patients from whom written informed consent was obtained |
| 3. Patients suspected of having malignant disease in peripheral lesions |
|
4. Patients with the following: PLT ≥50,000/mm3 ALT ≤150 IU/L AST ≤150 IU/L T-bil ≤1.5 mg/dL Cr ≤1.5 mg/dL 1.5<PT-INR<3.0 (Warfarin-only group) |
| Exclusion criteria |
| 1. Medical history of bleeding tendency or hemoptysis |
| 2. Concurrent use of steroid therapy |
| 3. Patients requiring a highly invasive procedure (cauterization or stent) |
| 4. Cases of emergency examinations |
| 5. Patients with active concomitant pregnancy |
| 6. Patients with serious drug allergic reactions. |
PLT: platelet count, ALT: alanine aminotransferase, AST: aspartate aminotransferase,
T.bil: total bilirubin blood, Cr: serum creatinine, PT-INR: prothrombin time-international standard ratio
Bronchoscopic Procedures
Experienced bronchoscopists performed the procedures with the assistance of at least two physicians and one nurse working in a bronchoscopy suit. The procedures performed included bronchial washing (BW), bronchial brushing (BB), and a transbronchial biopsy (TBB). Broncho-alveolar lavage, trans-bronchial needle aspiration, and endobronchial biopsies were not performed. The use of a guide sheath was not restricted.
Calculation of the Bleeding Volume
The bronchoscopist was informed of the screening history, clinical examination findings, and blood test results and carried out the necessary biological procedure at his or her own discretion. During the biopsy, any visible blood was aspirated into the specimen collection container through the bronchoscope. The volume of the mixed blood and lavage fluid (VM) aspirated was measured in milliliters. The hemoglobin concentration of this mixture (HbM) was measured using a portable hemoglobin measuring apparatus (HemoCue® Hb 201 +; HemoCue AB, Ängelholm, Sweden). The hemoglobin concentration of the patient’s blood (HbP) was determined as part of the full blood count before bronchoscopy. The amount of bleeding was calculated based on the formula used in a similar study (bleeding volume= VM×HbM / HbP) [ 12, 15].
Statistical Analyses
In first stage, if it became obvious from the data that the warfarin group had a very high occurrence rate of bleeding, we planned to end the study at that stage. All statistical analyses were conducted using the GraphPad Prism 7 software program (GraphPad Software, La Jolla, CA, USA). Median values were compared between two groups using the Mann-Whitney U-test. Differences in proportions were analyzed using Fisher’s exact test. A two-sided P value of <0.05 was considered significant.
Population Demographics and Disease Profiles
A total of 28 patients were included in the study ( Table 2). The control group was 15 men and 8 women, median age 70 years old, range 45–86 years old. The warfarin group was 4 men and 1 woman, median age 80 years old, range 69–86 years old. The reasons for taking warfarin were atrial fibrillation in three people, pulmonary embolism in one person, and deep vein thrombosis in one person. A large proportion of these subjects (78.6%, n = 22) were diagnosed with primary lung cancer. Inflammation was the second-most common disease investigated (14.2%, n = 4). The remainder of our subjects had nontuberculous mycobacteria (NTM) lung disease (7.1%, n = 2).
| Characteristics | Control (N=23) | Warfarin (N=5) | P-value |
|---|---|---|---|
| Age (years) | 70 (45-86) | 80 (69-86) | 0.082 |
|
Sex (N) Male/Female |
15/8 | 4/1 | >0.99 |
|
Disease profile (N) Lung cancer Inflammation NTM |
17 4 2 |
5 0 0 |
0.553 0.567 >0.99 |
|
Blood pressure (mmHg) Systolic blood pressure Diastolic blood pressure |
149 (105-198) 85 (70-114) |
148 (110-168) 83 (62-92) |
0.631 0.297 |
|
Laboratory data PT-INR PLT (×104/μl) Ht (%) ALT (IU/l) AST (IU/l) T-bil (mg/dl) Cr (mg/dl) |
0.96 (0.88-1.07) 23.8 (13.1-33.8) 40.4 (33.8-46.2) 18 (7-38) 22 (10-42) 0.70 (0.3-1.3) 0.68 (0.48-1.06) |
2.48 (1.65-2.85) 20.1 (18.6-33.1) 37.1 (35.0-46.5) 16.0 (9-22) 25.0 (18-35) 0.90 (0.4-1.4) 0.72 (0.65-0.95) |
0.001* >0.99 0.717 0.521 0.56 0.107 0.413 |
Data are presented as the median (range) or n.
NTM: nontuberculous mycobacteria, PT-INR: prothrombin time-international standard ratio, PLT: platelet count, Ht: hematocrit, ALT: alanine aminotransferase, AST: aspartate aminotransferase, T.bil: total bilirubin blood, Cr: serum creatinine
*: Statistically significant (p <0.05)
Laboratory Parameters
All laboratory parameters were assessed within six hours before flexible bronchoscopy ( Table 2). The control group had a median PT-INR of 0.96 (range 0.88–1.07), while the warfarin group had a median PT-INR of 2.48 (range 1.65–2.85), which was the optimal range. All 28 subjects had normal PLT, ALT, AST, T-bil, and Cr values.
Bronchoscopic Interventions
The median bronchoscopic procedure time was 40 (range 30–65) minutes in the control group and 39 (range 30–47) minutes in the warfarin group ( Table 3). A total of 27 subjects (96.4%) underwent BW, and 28 subjects (100%) received a TBB. TBBs were performed at least twice in all cases. The usage rate of the guide sheath was 74% (N = 17) in the control group and 100% (w = 5) in the warfarin group ( Table 3).
| Characteristics | Control (N=23) | Warfarin (N=5) | P-value |
|---|---|---|---|
| Time (min) | 40 (30-65) | 39 (30-47) | 0.380 |
|
Number of biopsies (times) Brushing TBLB |
2 (0-6) 6 (2-17) |
4 (1-6) 7 (3-8) |
0.067 0.684 |
| Diseased cases (N) | 21 | 5 | >0.99 |
|
Bronchoscopic interventions (times) TBB+BB TBB+BW TBB+BB+BW |
7 1 15 |
1 0 4 |
>0.99 >0.99 >0.99 |
|
Guide sheath (N) Used/Not used |
17/6 | 5/0 | 0.550 |
| Estimated blood loss (ml) | 0.2 (0-13.3) | 0.8 (0-1.2) | 0.641 |
Data are presented as the median (range) or n.
TBB: transbronchial biopsy, BB: bronchial brushing, BW: bronchial washing.
Bleeding Volume
In both groups of cases, no severe bleeding was recorded. In the control group, the median blood loss volume was 0.2 ml (range 0−13.3). In contrast, in the warfarin group, the median blood loss volume was 0.8 ml (range 0−1.2) ( Table 3, Fig. 1).
The estimated mean blood loss±95% confidence interval (CI) of the groups overlapped (Control: 0.45−3.49, Warfarin: −0.11−1.36). When the control group was divided based on whether or not a guide sheath had been used, its 95% CI completely overlapped with that of the warfarin group (Control −0.42−3.44, Warfarin −0.11−1.36) ( Fig. 2).
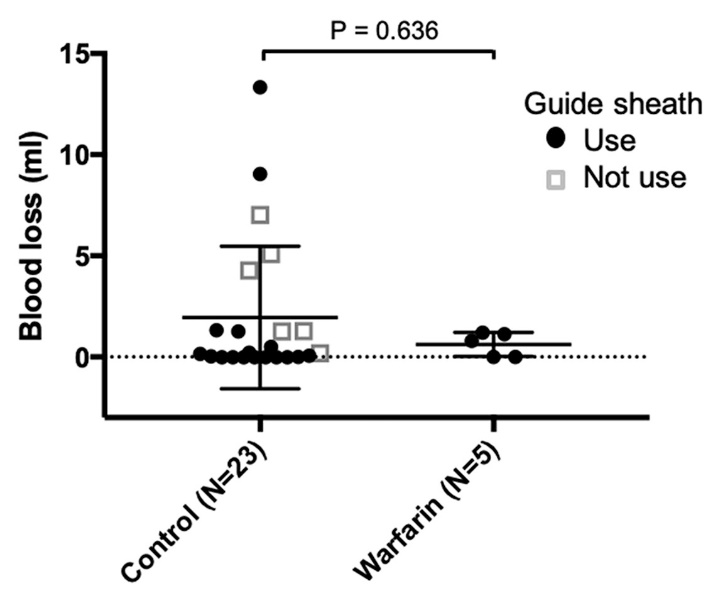
Multiple scatter plot with error bars of estimated blood loss. In the control group, the mean blood loss volume was 1.97 ml (range 0-13.3). In the warfarin group, the mean blood loss volume was 0.63 ml (range 0-1.2).
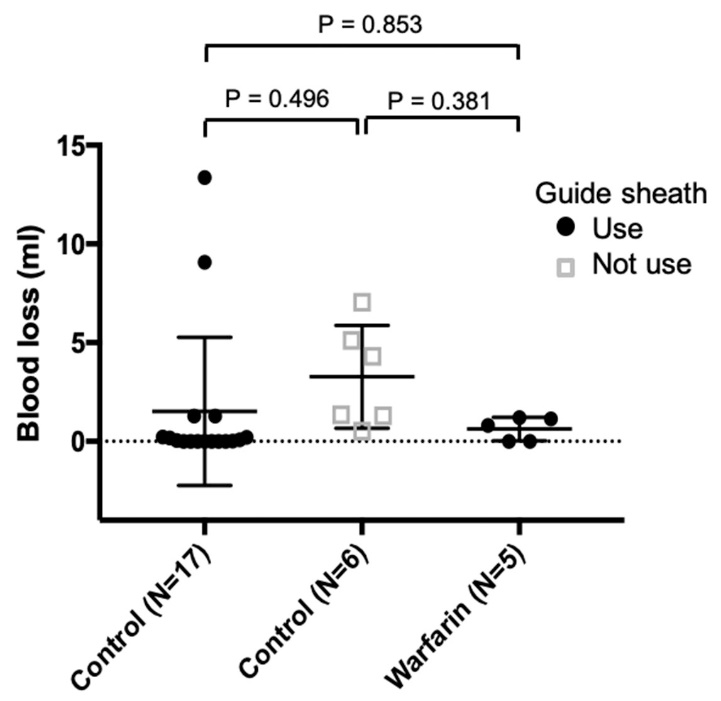
Multiple scatter plot with error bars of the estimated blood loss with and without a guide sheath.
Adverse Events
The onset of cerebral infarction and other thrombotic diseases was not observed in either group. All cases completed their bronchoscopic procedures.
The bleeding volume in this study was almost the same as in previous reports [ 12], which reported similar values for warfarin recipients. To reflect the influence of pure warfarin, cases with coagulation system abnormalities were excluded under the criteria. Initially, we planned to include 20 cases in the warfarin group, but this became impossible due to the spread of DOACs with few side effects of bleeding.
The use of DOACs in the field of oncology may be increased, reflecting venous thromboembolism (VTE) treatment in cancer patients [ 16, 17]. As there are no markers like PT-INR, this study did not include DOACs. Since it is supposed that side effects of bleeding are originally small, we plan to conduct a study involving anticoagulants, including DOACs, in the future.
However, warfarin is actively used in patients with valvular atrial fibrillation, as well as elderly and renal dysfunction patients. Some patients wish to receive warfarin for financial reasons, as low-molecular-weight heparin for VTE is not covered by insurance in Japan. Therefore, patients who require warfarin are likely to continue to exist to some degree.
Whether or not bridging anticoagulation (low-molecular-weight heparin) is necessary for patients who require warfarin treatment interruption for an invasive procedure is unclear. In recent years, there have been an increasing number of negative reports suggesting that bridging anticoagulation is associated with higher risk for bleeding during warfarin therapy interruption for invasive procedures without diminishing to the onset of embolism [ 6- 8]. Bronchoscopy is conventionally performed in short-term hospitalization or as an outpatient service. However, bridging anticoagulation (low-molecular-weight heparin) takes about 5−7 days to complete. Therefore, if warfarin continuation is deemed acceptable, such issues will be solved.
Regarding the hemostasis procedure after the biopsy, by keeping the guide sheath for a while, the same action as the compression hemostatic can be expected. The present study was the first clinical trial to target patients, and lesions were limited to peripheral lesions with the use of a guide sheath. Our findings do not guarantee the safety of such an approach for central lesions, so further examinations will need to be conducted after continuing case collection.
Bronchoscopy is required not only as a diagnostic tool for lung cancer but also as a means of determining treatment policy, not necessarily only once in life. The importance of bronchoscopy in the respiratory diagnosis is increasing, as are cases in which antithrombotic drugs cannot be discontinued thanks to the spread of new therapies, such as drug-eluting stents.
It has been reported in several studies that the interruption of warfarin causes cerebral infarction and other thrombotic diseases at a frequency of about 1%, and many cases are severe [ 18, 19]. There is also a report that the risk of cerebral infarction recurrence rises 3.4-fold when antiplatelet drugs are interrupted in a patient with cerebral infarction [ 20, 21]. Given the present clinical conditions, the risk of thromboembolism whenever anti-thrombotic drugs are withdrawn should be carefully considered.
Our study is limited by its small sample size. However, because this was a single-center study, we were able to reduce the variability in the procedure. We hope that this research will prove a useful source to consider for developing into a multicenter study. Furthermore, this analysis did not include bronchoalveolar lavage, transbronchial needle aspiration, or endobronchial biopsy cases, so the amount of blood loss was not examined for these procedures.
Although limited to peripheral lesions and cases with a guide sheath, we found no severe bleeding in the warfarin group. It may be acceptable for warfarin to be continued when performing flexible bronchoscopy.
Conflict of interest statement
Takeshi Isobe received honoraria from Boehringer-ingelheim, Astrazeneca, and Pfizer. Yukari Tsubata received honoraria from Astrazeneca, Daiichi Sankyo Co., and Chugai Pharmaceutical Co.
Acknowledgements
None.