Abstract
Background: Major depression (MD) is the most prevalent mood disorder worldwide. Electroconvulsive treatment (ECT) is a highly effective treatment in psychiatry and shows efficacy in patients who were resistant to pharmacotherapy. Recent retrospective cohort studies have shown that ECT is highly effective in ameliorating depressive symptoms. Similar ECT efficacy has been established also in various rodent models of MD. To establish further efficacy of ECT in rodent depression models, we investigated the effect of ECT on depressive-like behaviour induced by lipopolysaccharide (LPS) in rats. Methods: Adult male Sprague-Dawley (SD) rats were randomly divided into four groups, namely, control, LPS, sham ECT, and LPS + ECT. The SD rats received intraperitoneal injection of LPS or sterile saline, followed by ECT or sham treatment for 7 consecutive days. Subsequently, the forced swimming test (FST) and Y-maze test were performed. Results: The FST showed that the immobility time in the LPS group (169.73 ± 15.46 s) was significantly longer than that in the control group (63.16 ± 4.48 s). However, ECT administration to LPS-injected rats significantly shortened the prolonged immobility. The Y-maze test showed a significant decrease in % spontaneous alternation behaviour (SAB) in the LPS group compared to the control group. ECT administration to LPS-injected rats significantly restored such a decrease in % SAB. Conclusions: Our results suggest that repeated ECT ameliorated LPS-induced depressive-like behaviour in SD rats. Further studies are warranted to elucidate the molecular mechanism of such therapeutic effects of ECT.
Abbreviations: MD, major depression; ECT, electroconvulsive treatment; LPS, lipopolysaccharide; FST, forced swimming test; SAB, spontaneous alternation behaviour
INTRODUCTION
Major depression (MD) is the most prevalent mood disorder worldwide [1]. This mental disorder has increased social burden with a significant proportion of mortality [2]. MD affects about 280 million people worldwide [3]. The influences of MD can be persistent or recurrent and can dramatically affect a person’s ability to function and live a rewarding life [4].
Electroconvulsive treatment (ECT) is a highly effective treatment in psychiatry and shows efficacy in patients who were resistant to pharmacotherapy [5, 6]. ECT elicits substantial improvement in approximately 80% of patients [7, 8]. Both the generalized seizure and the dose of electricity used are believed to be critical for the therapeutic effect of ECT [9]. However, the exact mechanism of therapeutic action of ECT remains unknown.
Recent retrospective cohort studies have shown that ECT is highly effective in ameliorating depressive symptoms with fewer unwanted effects [6, 10]. Similar ECT efficacy has been established also in depression rodent models, such as MAP6 KO mice [11] and rats under chronic restraint stress [12]. To establish further efficacy of ECT in rodent depression models, we investigated the effect of ECT on depressive-like behaviour induced by lipopolysaccharide (LPS) in rats. LPS is a well-establish proinflammatory stimulant to induce depressive-like behaviour in rodents [13, 14]. The present study shows that ECT ameliorates LPS- induced depressive-like behaviour in rats.
MATERIALS AND METHODS
Animals
Twenty adult male Sprague-Dawley (SD) rats, six-week-old were employed. The rats were housed in groups under standard conditions, namely, 12 h light/12 h dark cycle (lights on from 7:00 to 19:00) at a room temperature (RT) of 23 ± 2℃ with a humidity level of 55 ± 5%. One week before commencing experiments, the rats undertook a handling procedure once a day to reduce experimental stress. The experimental procedures were conducted with the approval of the Shimane University Animal Ethics Committee (Authorization No: IZ31-37), in accordance with the guidelines of the National Health and Medical Research Council of Japan.
Reagents
LPS of Escherichia coli (serotype 055: B5; Sigma-Aldrich) was dissolved into fresh sterile saline (0.9% NaCl) prior to the administration. All intraperitoneal (i.p.) injections were administered at a volume of 1 ml/kg body weight. The dosage of LPS was determined based on results of preliminary tests that examines the LPS effect on rat behaviour.
Intraperitoneal injection procedure
The SD rats received once-daily i.p. injection of LPS solution or an equal volume of sterile saline for 7 consecutive days. After identifying the anatomical landmarks of the rat, disinfected through gauze with 70% alcohol. To avoid hitting such organs as the liver, bladder, and cecum of rats, i.p. injection was administrated into the lower right quadrant of the abdomen.
ECT procedure
The SD rats received once-daily ECT or sham treatment for 7 consecutive days. To avoid stress or pain induced by the ECT procedure, each rat was first anesthetized in an isoflurane inhalation chamber (4% for initial induction) with an oxygen flow rate of 2–4 L/min. After the rat fell asleep, it was taken out of the chamber, and anesthesia was continued by putting an isoflurane inhalation mask (2% for maintenance) with an oxygen flow rate of 2–4 L/min. In every ECT, an electric shock was given under such anesthesia. ECT was administered transcranially via bilateral ear clip electrodes by using an E.C. Stimulator MK-810 (Muromachi Kikai, Tokyo, Japan). The stimulus was a sine wave pulse, 100 V, 60 Hz, 50 mA for 1.5 s. Each stimulation resulted in a typical tonic-clonic seizure lasting for less than 10 s. The rats in the sham ECT groups received sham ECT treatment, including isoflurane anesthesia and placement of the ear clip electrodes without delivery of the electroconvulsive shock.
Experimental design
Experiments were carried out during the light period. The rats were randomly divided into four groups (n = 5): control (i.p. injection of saline), LPS (i.p. injection of LPS), sham ECT (i.p. injection of saline + sham ECT), and LPS + ECT group (i.p. injection of LPS + ECT). The i.p. injections of either LPS or an equal volume of sterile saline was carried out once daily in the morning (8:00 to 9:00) from day 8 to 14 (Fig. 1). ECT was administered 2 hours after the i.p. injection of LPS. Body weight was monitored and recorded every day before i.p. injection. The Y-maze test and habituation of forced swimming test were performed on day 14 after the last ECT and the forced swimming test (FST) was performed on the next day. After completing the behaviour tests, the animals were killed by the overdose of Carbon dioxide (CO2) in the specific container.
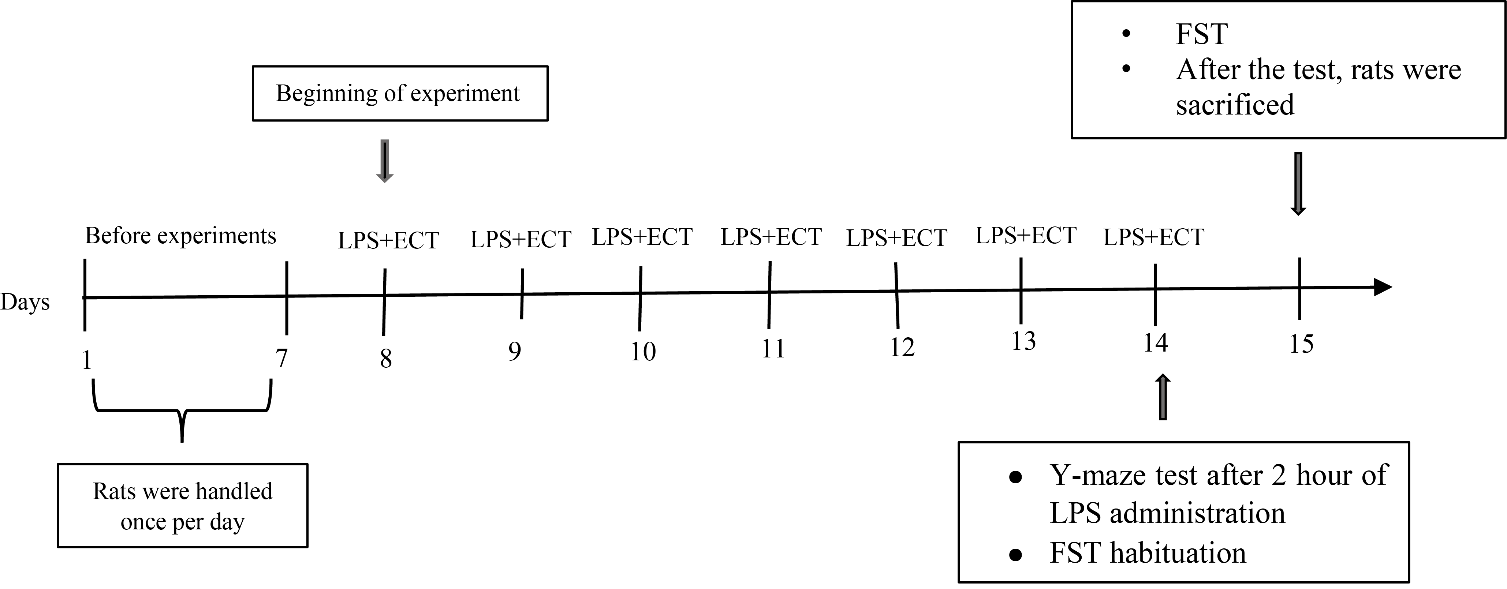
Figure 1. Experimental design. Once-daily procedure of LPS i.p. injection and ECT was performed for consecutive 7 days. After the last LPS injection and ECT, the Y-maze test and the forced swim test were performed.
Behavioural tests
Each SD rat received the behaviour tasks one by one. In the following description order, the first Y-maze test, and the habituation of the forced swimming test was performed on day 14 and a real forced swimming test was performed on day 15. All the tests were carried out between 12:00 and 14:00. The behavioural tests were monitored and recorded by a digital camera.
Forced swimming test (FST)
The forced swimming test seems suitable for detecting antidepressant-like behaviour activity in rats but not mice [15]. The swim test involves the counting of active (swimming and climbing) or passive (immobility) behaviour when rodents are forced to swim in a cylinder from which there is no escape [16]. The FST was performed as previously reported with some modifications [13, 17, 18, 19]. Each rat was placed individually and gently in a plastic cylinder (diameter 19 cm) filled with 10 L water (temperature 25 ± 2℃, depth 40 cm) for two consecutive days. The rat was unable to touch the top and bottom of the cylinder. After completing each trial, the rat was dried with a towel and placed in an incubator to prevent hypothermia, then returned to the home cage. In addition, the cylinder was washed, dried, and refilled with water to avoid impact on subsequent animals. The rat was gently placed in water for 15 minutes as habituation on the first day. The next day, the rat was gently placed in the water and its behaviour was recorded on a video camera for 6 minutes. The swimming, climbing, and immobility time that occurred during the test was calculated manually by a blind-trained observer. Rats were considered immobile when they remained afloat without struggling, were less moving, and move only to keep their heads above the surface of the water. Immobility time can be regarded as behavioural despair which is supposed to reflect depressed moods. [20].
Y-maze test
The spatial working memory of rats was considered by the Y-maze test after 2 hours of the last ECT administration based on previously described procedures with some modifications [18, 21]. The maze apparatus is made of grey opaque polyvinyl chloride with three arms (500 × 250 × 100 mm3 and 120° apart) (Muromachi Kikai). The apparatus was cleaned with a 10% ethanol solution and then dried with a paper towel after each trial to make it odorless. Each rat was placed in the middle of one arm and allowed to move freely through the maze for 8 min. When the rat’s tail was entirely within the arm, entry was considered to be complete. Alternation behaviour was defined as successive entries into all three arms on consecutive occasions. The percentage of spontaneous alternation behaviour (SAB) was calculated according to the following formula:
%SAB
=
The number of alternation
The total number of arm entries
-
2
×
100
%
Statistical Analysis
All data were presented as the mean ± standard error of the mean (S.E.M.). We used a one-way analysis of variance followed by a post hoc Tukey’s honestly significant difference test to evaluate the differences among groups. The statistical analyses were performed with SPSS software (IBM SPSS Statistics for Windows Version 23, SPSS Japan Inc., Tokyo, Japan). The p-value was considered as significant when less than 0.05.
RESULTS
A change in body weight after experimental procedure
We measured body weight to determine whether ECT ameliorates appetite loss, which is one of prominent symptoms of MD. The average body weight was 263.43 ± 6.89 g in the control group, 258.80 ± 6.20 g in the sham ECT group, 234.26 ± 8.57 g in the LPS group, and 236.20 ± 5.04 g in the LPS + ECT group (Fig. 2). The rat body weight in the LPS group was significantly lower than that of control rats. ECT did not significantly increase the rat body weight in the LPS group (Fig. 2). These findings suggest that ECT does not restore the appetite loss caused by LPS injection.

Figure 2. The effects of ECT on body weight of SD rats. Each value is the mean ± SEM. n=5, *p < 0.05, n.s., not significant, ECT, Electroconvulsive treatment, LPS, lipopolysaccharide.
Effect of ECT on immobility time in the FST
To evaluate the effect of ECT on depressive-like behaviour in LPS-injected rats, we measured immobility time in the FST. The immobility time in the LPS group was 169.73 ± 15.46 s, which was significantly longer than those in the control group (63.16 ± 4.48 s) and the sham ECT group (70.97 ± 10.52 s) (Fig. 3). However, ECT administration to LPS-injected rats significantly shortened the prolonged immobility time (Fig. 3). ECT on non-injected rats did not affect immobility time. These findings suggest that ECT improves LPS-induced depressive-like behaviour.

Figure 3. The effects of ECT on immobility time in the forced swim test. Each value is the mean ± S.E.M. n=5, *p < 0.05, n.s., not significant, ECT, Electroconvulsive treatment, LPS, lipopolysaccharide.
Effect of ECT on climbing time and swimming time in the FST
Climbing time in the LPS group (43.62 ± 4.20 s) was significantly shorter than the control group (73.06 ± 7.35 s) and sham ECT group (68.16 ± 3.45 s) (Fig. 4). However, ECT on LPS-injected rats significantly prolonged the climbing time (Fig. 4).
Swimming time in the LPS group (136.44 ± 9.66 s) was significantly shorter than that in the control group (232.22 ± 5.74 s) and sham ECT group (222.87 ± 9.81 s) (Fig. 5). Nevertheless, ECT on LPS-injected rats significantly lengthened the swimming time (Fig. 5). These findings also suggest that ECT improves LPS-induced depressive-like behaviour.
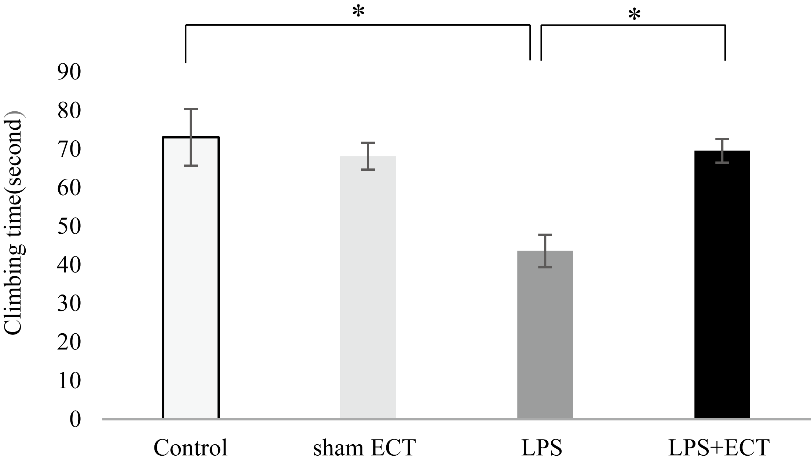
Figure 4. The effects of ECT on climbing time in the forced swim test. Each value is the mean ± S.E.M. n = 5, *p < 0.05, n.s., not significant, ECT, Electroconvulsive treatment, LPS, lipopolysaccharide.
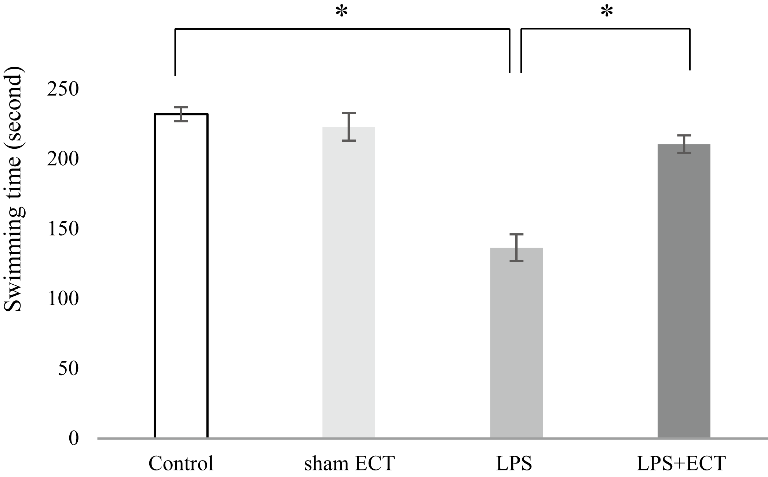
Figure 5. The effects of ECT on swimming time in the forced swim test. Each value is the mean ± S.E.M. n=5, *p < 0.05, n.s., not significant, ECT, Electroconvulsive treatment, LPS, lipopolysaccharide.
Effect of ECT on spontaneous alternation behaviour in the Y-maze test
Spatial working memory was examined by SAB in the Y-maze test. The percentage of SAB was 73.55 ± 2.73% in the control group, 66.86 ± 3.24% in the sham ECT group, 51.72 ± 3.95% in the LPS group, and 69.27 ± 4.10% in the LPS + ECT group (Fig. 6). A significant decrease in % SAB was observed in the LPS group compared to the control group (Fig. 6). ECT administration to LPS-injected rats significantly increased % SAB (Fig. 6). This finding suggests that ECT improved the spatial memory impairment in rats with LPS-induced depressed-like behaviour.

Figure 6. The effects of ECT on spontaneous alternation behaviour (SAB) in the Y-maze test. Each value is the mean ± S.E.M. n=5, *p < 0.05. SAB, spontaneous alternation behaviour; ECT, electroconvulsive treatment.
DISCUSSION
There are two major findings in the present study. First, ECT significantly reduced the prolonged immobility time in rats injected with LPS in the FST. Second, ECT significantly increased a decrease in % SAB in LPS-injected rats in the Y-maze test. These findings suggest that ECT significantly ameliorate the LPS-induced depressive-like behaviour, including despair and impaired spatial working memory. To our knowledge, this is the first study showing the therapeutic effects of ECT on LPS-induced depressive-like behaviour in rats.
The first finding is consistent with the previous studies of depressive rodent models using FST, in which ECT significantly shortened immobility time in MAP6 KO mice [11] and in rats under chronic restraint stress [12]. Accordingly, ECT may exert therapeutic effects even in different pathogeneses associated with MD. Immobility time in the FST can be regarded as behavioural despair, which is supposed to reflect depressed moods. The FST on rodents has been extensively used as a simple animal model for investigating the neurobiology of MD because of its procedural simplicity and its high reproducibility [22, 23, 24].
MD often impairs working memory, while ECT has adverse effects such as cognitive deficit [25]. This clinical observation has led to a debate as to whether cognitive deficit after ECT is side effects of ECT or an intrinsic pathological component of major depression. Our result of improvement of spatial working memory impairment after ECT suggests that cognitive deficit is causally associated with MD.
In this study, we employed an inflammation-induced depression model to determine ECT efficacy. Yirmiya et al. (1996) first demonstrated that the systemic administration of LPS caused depressive-like behavior in rats [26]. A number of animal studies have demonstrated that systemic inflammation induced by peripheral administration of LPS increases the expression of pro-inflammatory cytokines in both the periphery and brain and causes abnormal behaviour similar to MD [14, 27, 28]. It is not entirely clarified the manner in which the periphery communicates with the brain, modulates neurotransmission, and consequently changes behaviour. Nevertheless, several routes of communication between the systemic immune system and the brain are postulated [reviewed in 29 and 30].
Increasing evidence suggests that infection and persistent low-grade inflammation in peripheral tissues play roles in the MD pathogenesis [30]. Periodontitis is the most common inflammatory disease in adults. Accordingly, treating periodontitis may be therapeutic for MD.
It is clinically known that many people begin to notice an improvement in their symptoms after about six treatments with electroconvulsive therapy [31]. Our result showed that ECT performed for consecutive 7 days improved depressive-like behaviour. Therefore, our experimental condition of ECT seems relevant to a clinical setting.
We also found that ECT did not significantly affect the decreased body weight after LPS injection. Appetite loss is one of the prominent MD symptoms. It is clinically recognised that that ECT can improve such appetite loss. It is, nevertheless, unknown why ECT under our experimental condition did not restore the LPS-caused body weight loss.
In conclusion, we demonstrated that repeated ECT ameliorated LPS-induced depressive-like behaviour in SD rats. Further studies are clearly warranted to elucidate the molecular mechanism of such therapeutic effects of ECT.
REFERENCES
- 1) Gutiérrez-Rojas L, Porras-Segovia A, Dunne H, Andrade-González N, Cervilla JA. Prevalence and correlates of major depressive disorder: a systematic review. Braz J Psychiatry 2020;42:657-672. doi: 10.1590/1516-4446-2020-0650.
- 2) Walker ER, McGee RE, Druss BG. Mortality in Mental Disorders and Global Disease Burden Implications: a systematic review and meta-analysis. JAMA Psychiatry 2015;72:334-341. doi: 10.1001/jamapsychiatry.2014.2502.
- 3) Searing L. Depression affects about 280 million people worldwide. The Washington Post. https://www.washingtonpost.com/health/2022/02/27/depression-worldwide/. (updated February 27, 2022).
- 4) World Health Organization (WHO). Depression. World Health Organization. https://www.who.int/health-topics/depression#tab=tab_1. (accessed September 13, 2021).
- 5) Husain MM, Rush AJ, Fink M, et al. Speed of response and remission in major depressive disorder with acute electroconvulsive therapy (ECT): a Consortium for Research in ECT (CORE) report. J Clin Psychiatry 2004;65:485-491. doi: 10.4088/jcp.v65n0406.
- 6) Karayagmurlu A, Coskun M, Elboga G, et al. Efficacy and safety of electroconvulsive therapy in adolescents: a retrospective chart review study from turkey. J ECT 2020;36(1):54-59. doi: 10.1097/YCT.0000000000000602.
- 7) Kerner N, Prudic J. Current electroconvulsive therapy practice and research in the geriatric population. Neuropsychiatry (London). 2014;4(1):33-54. doi: 10.2217/npy.14.3.
- 8) Spaans HP, Sienaert P, Bouckaert F, et al. Speed of remission in elderly patients with depression: electroconvulsive therapy v. medication. Br J Psychiatry 2015;206:67-71. doi: 10.1192/bjp.bp.114.148213.
- 9) Anderson IM, Fergusson GM. Mechanism of action of ECT. In: Waite J, Easton, editors. A the ECT handbook. 3. London: Royal College of Psychiatrists; 2013:1-7.
- 10) Liang CS, Chung CH, Tsai CK, Chien WC. In-hospital mortality among electroconvulsive therapy recipients: A 17-year nationwide population-based retrospective study. Eur Psychiatry 2017;42:29-35. doi: 10.1016/j.eurpsy.2016.12.005.
- 11) Jonckheere J, Deloulme JC, Dall’Igna G, et al. Short- and long-term efficacy of electroconvulsive stimulation in animal models of depression: The essential role of neuronal survival. Brain Stimul 2018;11:1336-1347. doi: 10.1016/j.brs.2018.08.001.
- 12) Olesen MV, Wortwein G, Folke J, Pakkenberg B. Electroconvulsive Stimulation Results in Long-Term Survival of Newly Generated Hippocampal Neurons in Rats. Hippocampus 2017;27:52-60. doi: 10.1002/hipo.22670.
- 13) Jaya MA, Hayashida M, Tsuchie K, et al. Effect of Ninjinʼyoeito on Lipopolysaccharide-Induced Depressive Like Behavior and Glial Activation in the Hippocampus. Shimane J Med Sci 2022;39:1-13. doi: 10.51010/sjms.39.1_1.
- 14) O’Connor JC, Lawson MA, Andre C, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry 2009;14:511-522. doi: 10.1038/sj.mp.4002148.
- 15) Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147-160. doi: 10.1007/BF00176837.
- 16) Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc 2012;7(6):1009-14. doi: 10.1038/nprot.2012.044.
- 17) Yankelevitch-Y R, Franko M, Huly A, Doron R. The forced swim test as a model of depressive-like behavior. J Vis Exp 2015;97:52587. doi: 10.3791/52587.
- 18) Azis IA, Hashioka S, Tsuchie K, et al. Electroconvulsive shock restores the decreased coverage of brain blood vessels by astrocytic endfeet and ameliorates depressive-like behavior. J Affect Disord 2019;257:331-9. doi: 10.1016/ j.jad.2019.07.008.
- 19) Arauchi R, Hashioka S, Tsuchie K, et al. Gunn rats with glial activation in the hippocampus show prolonged immobility time in the forced swimming test and tail suspension test. Brain Behav 2018;8:e01028. doi: 10.1002/brb3.1028.
- 20) Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The Mouse Forced Swim Test. J Vis Exp 2012;59:e3638. doi: 10.3791/3638.
- 21) Shi L, Zhang Z, Li L, Holscher C. A novel dual GLP-1/GIP receptor agonist alleviates cognitive decline by re-sensitizing insulin signalling in the Alzheimer icv. STZ rat model. Behav Brain Res 2017;327:65-74, doi: 10.1016/j.bbr.2017.03.032.
- 22) Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature 1977;266:730-732. doi: 10.1038/266730a0.
- 23) Bogdanova OV, Kanekar S, D’Anci KE, Renshaw PF. Factors influencing behavior in the forced swim test. Physiol Behav 2013;118:227-239. doi: 10.1016/j.physbeh.2013.05.012.
- 24) Porsolt RD. Animal models of depression: utility for transgenic research. Rev Neurosci 2000;11(1):53-8. doi: 10.1515/revneuro.2000.11.1.53.
- 25) Vasavada MM, Leaver AM, Njau S, et al. Short- and long-term cognitive outcomes in patients with major depression treated with electroconvulsive therapy. J ECT 2017;33:278-285. doi: 10.1097/YCT.0000000000000426.
- 26) Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res 1996;711:163-74. doi: 10.1016/0006-8993(95)01415-2.
- 27) Frenois F, Moreau M, O’Connor J, et al. Lipopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology 2007;32:516-531. doi: 10.1016/j.psyneuen.2007.03.005.
- 28) Biesmans S, Meert TF, Bouwknecht JA, et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediators Inflamm 2013;2013:271359. doi: 10.1155/2013/271359.
- 29) D’Mello C, Swain MG. Immune-to-brain communication pathways in inflammation-associated sickness and depression. Curr Top Behav Neurosci 2017;31:73-94. doi: 10.1007/7854_2016_37.
- 30) Hashioka S, Inoue K, Hayashida M, Wake R, Oh-Nishi A, Miyaoka T. Implications of Systemic Inflammation and Periodontitis for Major Depression. Front Neurosci 2018;12:483. doi: 10.3389/fnins.2018.00483.
- 31) Mayo Foundation for Medical Education and Research (MFMER). Mayo Clinic. https://www.mayoclinic.org/tests-procedures/electroconvulsive-therapy/about/pac-20393894. (accessed Oct. 12, 2018).






