2022 年 39 巻 4 号 p. 113-121
2022 年 39 巻 4 号 p. 113-121
Carotid artery stenosis is the major cause of stroke. Carotid artery stenting (CAS) is the less invasive treatment for this condition. But, the tortuosity of aortic arch or carotid bifurcation are considered a risk factor for CAS. The authors evaluated the impact of catheter access route on outcomes. A retrospective study was conducted on patients with CAS from 2015 to 2020. Accessed anatomical factors were acute angle type of aortic arch and carotid artery angulation. The outcomes were set as symptomatic stroke within 30 days postoperatively and postoperative ischemic diffusion-weighted imaging (DWI) lesions. In this study, 157 cases were included. In bivariate analysis, left-sided lesions and symptomatic stroke (p = 0.028), as well as age and ischemic DWI lesions (p = 0.004), were associated. On the other hand, two anatomic factors did not affect post-treatment outcomes in bivariate analysis. Safe treatment can be expected by preoperative evaluation of vascular anatomy.
The cervical internal carotid artery (ICA) is prone to arterial stenosis caused by atherosclerosis, and when the ICA stenosis (ICS) is severe, it can cause cerebral infarction. Randomized controlled trials (RCTs) have studied the therapeutic efficacy of pharmacological versus surgical therapies to prevent stroke caused by severe ICS. In the 1990s, two RCTs, NASCET [1], ACAS [2], demonstrated the superiority of surgical therapy over medical treatment in symptomatic ≤50% stenotic lesions and asymptomatic ≤70% stenotic lesions. Carotid endarterectomy (CEA) for these lesions was widely performed since these studies in world wide. In the 2000s, carotid artery stenting (CAS), a minimally invasive catheter-based treatment, was introduced. The SAPPHIRE study [3] demonstrated the superiority of CAS over CEA for older adults and those at high risk for surgical procedures such as cardiac and respiratory disease. Since SAPPHIRE, five RCTs of CEA vs CAS (EVA-3S [4], SPACE [5], ICSS [6], CREST [7], ACT-1 [8]) have been published. These studies reported that while CAS was non-inferior to CEA, CAS resulted in more perioperative minor strokes. Perioperative ischemic complications are thought to be influenced by distal embolic protection techniques for CAS, and various embolic protection devices are currently being used to reduce intraoperative ischemic complications.
Two studies, SPACE and CREST, also reported a higher risk of perioperative ischemic stroke in older adults undergoing CAS. The results of these studies led to a revalidation of the usefulness of CAS for older adults as reported in the SAPPHIRE study. Anatomic risk factors have been shown to contribute to this, including severe angulation of the descending aorta and aortic arch, as well as the common carotid artery (CCA) and ICA [9–11].
Our facility is actively performing CAS as a surgical treatment for ICS in older adults because it is less invasive and can be performed under local anesthesia. In the present study, we examined how the anatomic factors of severe angulation of the aortic arch and carotid lesion affect the outcome of CAS.
Patients Population
Patients diagnosed with cervical ICS and who underwent CAS at Shimane University Hospital between January 2015 and December 2020 were included in the study. Dissected lesions, perioperative re-treatment after CAS, restenotic lesions after CAS, and CAS performed emergently were excluded. In addition, the transfemoral artery approach was included in this study; patients who underwent the procedure via the transbrachial approach were excluded.
Treatment
Prior to treatment, CAS patients have oral aspirin (100 mg) and clopidogrel (75 mg) tablet once daily for 14 days. Preoperative laboratory tests included blood tests, ultrasound of the carotid artery, simple computed tomography (CT) of the cervical region, and magnetic resonance angiography (MRA) or contrast-enhanced CT angiography (CTA) to access the aortic arch and cerebral vessels.
CAS was performed under local anesthesia via the transfemoral approach. Balloon guiding catheter and distal filter protection device (FilterWires EZ; Stryker, Kalamazoo, MI, USA) were used as embolic protection devices in all patients. Balloon protection (PercuSurge GuardWire; Medtronic, Minneapolis, MN, USA) was added at the oriffice of the external carotid artery (ECA) at the discretion of the surgeon. Open-cell stent (Cordis PRECISE® Pro RX; Cordis Endovascular, Johnson & Johnson) was used in all patients.
After surgery, oral aspirin (100 mg) and clopidogrel (75 mg) tablet was continued for 30 days. Magnetic resonance imaging (MRI) was performed within one week after surgery to detect the postsurgical ischemic lesions.
Evaluation items
Data collected included sex, age, side of lesion, type of the aortic arch, bifurcation angle between CCA and ICA (CCA-ICA angle) (Fig. 1), degree of stenosis and calcification of the stenotic lesion, preoperative renal function, and medical history. The success or failure of CAS was also assessed by two factors: A) symptomatic ischemic stroke within 30 days of surgery and B) postoperative ischemic DWI lesions.
Stenotic lesion is measured by ultrasound using the European Carotid Surgery Trial (ECST) method and area stenosis rate (Area). The degree of calcification on the lesion is measured by CT and differentiated by whether it is more than 1/2 circumference. The shape of the aortic arch was classified by the relationship between the aortic arch and brachiocephalic artery origin height using MRA or CTA (Type I, II, and III [12]), or the presence of a bovine arch (Fig. 2). Acute angle type of aortic arch was defined as a type III for right-sided stenotic lesions, and as a type III or bovine arch for left-sided stenotic lesions. CCA-ICA angle is measured from the angiography during procedure. Preoperative renal function is assessed by estimated glomerular filtration rate (eGFR). Medical history was reviewed for diabetes, hypertension, hyperlipidemia, and smoking history. Postoperative MRI was used to classify the presence of postoperative ischemic DWI lesions.

CCA, common carotid artery; ICA, internal carotid artery; ECA, external carotid artery
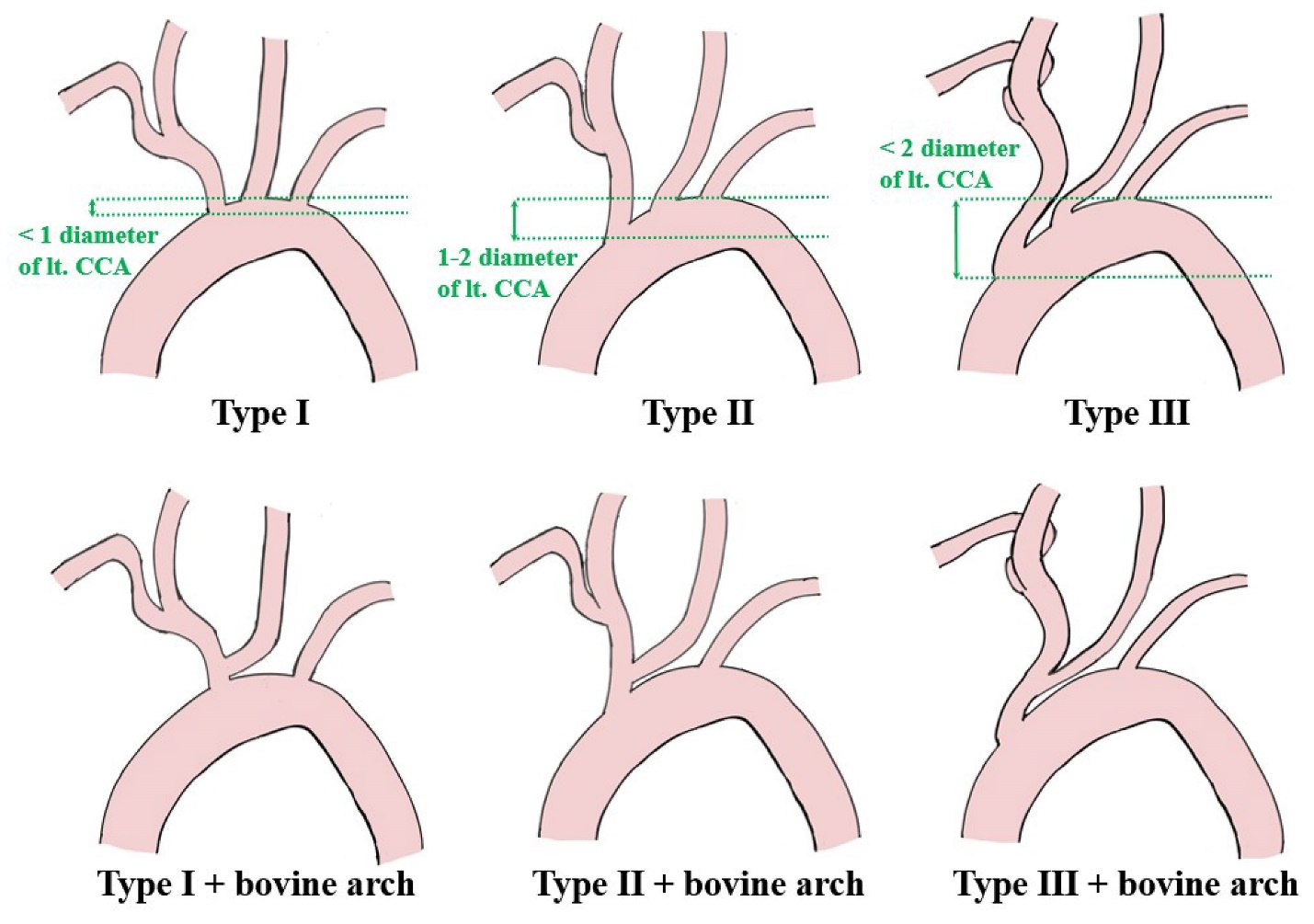
Schema of aortic aorta classification type I–III and bovine arch [10]
CCA, common carotid artery
Statistical analysis
Values are presented as medians. Categorical variables were compared with Fisher's exact probability test. Continuous variables with normal distributions were analyzed with Student's t-test and non-normally distributed ones with Mann-Whitney U-test. P values less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed using JMP® Pro version 16 (SAS Institute, Cary, NC).
A total of 186 cases of CAS were performed. Thirteen cases were excluded for re-treatment or additional surgery for restenosis or plaque protrusion after stenting, eight cases were excluded for emergency surgery for acute occlusion or dissecting lesions, and five cases were excluded for transbrachial approach. Three cases in which MRI imaging was not performed because of pacemaker implantation were excluded because postoperative cerebral ischemic findings could not be evaluated. The final number of cases incorporated was 157 (Fig. 3).
A total of 157 cases were applicable to this study, with males accounting for 136 (86.6%). The median age was 75 years, and 88 cases (56.1%) were 75 years or older. Clinical data for all cases included in this study are shown in Table 1. Left side lesions were 63 cases (40.1%), and the median stenosis rate was 75% by ECST and 85% by area methods, with more than half of the cases having severe stenosis. Lesion calcification > 1/2 was observed in 31 cases (19.7%). In the aortic arch, 68 (43.3%), 52 (33.1%), and 37 (23.6%) cases had Type 1, 2, and 3 arteries, respectively, and 27 (17.2%) cases had bovine arch. Acute angle type of aortic arch was present in 48 cases (30.6%). Of the 94 right-sided lesions, 26 (27.7%) were acute angle type aortic arch. Of the 63 left-sided lesions, 22 (34.9%) were acute angle type aortic arch. The median CCA-ICA angle was 148°, with 13 cases (8.3%) having a very acute angle of 120° or less. The median eGFR was 66 mL/min/1.73 m2, and 50 patients (31.8%) had an eGFR less than 60 mL/min/1.73 m2. Medical history included hypertension in 123 (78.0%), diabetes in 72 (45.9%), hyperlipidemia in 117 (74.5%), and smoking in 104 (66.2%). There were 15 (9.6%) symptomatic stroke cases within 30 days after surgery, of which 12 (7.6%) consisted of minor stroke and 3 (1.9%) of major stroke. Eighty-seven cases (55.4%) had postoperative ischemic DWI lesions. All 15 cases with postoperative symptomatic stroke had postoperative ischemic DWI lesions.
Symptomatic ischemic stroke within 30 days after surgery and bivariate analysis of each item are shown in Table 2. An association with left-sided lesions was suggested (p = 0.028). On the other hand, the results showed that sex (p = 0.422), age (p = 0.234), ECST (p = 0.612), Area (p = 0.153), lesion calcification > 1/2 (p = 0.181), acute angle type of aortic arch (p = 0.807), the CCA-ICA angle (p = 0.911), eGFR (p = 0.974), hypertension (p = 0.411), diabetes (p = 0.541), hyperlipidemia (p = 0.609) and smoking history (p = 0.971) were not associated.
Next, Table 3 shows a bivariate analysis of postoperative ischemic DWI lesions and each item. A significant association with age was suggested (p = 0.004). Meanwhile, there was no association with sex (p = 0.440), lesion side (p = 0.312), ECST (p = 0.835), Area (p = 0.622), lesion calcification > 1/2 (p = 0.635), acute angle type of aortic arch (p = 0.125), CCA-ICA angle (p = 0.971), eGFR (p = 0.971), hypertension (p = 0.743), diabetes (p = 0.723), hyperlipidemia (p = 0.759) or smoking history (p = 0.056).
DWI, diffusion-weighted imaging.
| No. | Total 157 |
|---|---|
| Sex male | 136/157 (86.6%) |
| Age (years), median (IQR) | 75 (70, 81) |
| Lt. side | 63/157 (40.1%) |
| Lesion stenosis | |
| ECST (%), median (IQR) | 75 (65, 82) |
| Area (%), median (IQR) | 85 (79, 90) |
| Lesion calcification > 1/2 | 31/157 (19.7%) |
| Aortic arch classification | |
| Type I | 68/157 (43.3%) |
| Type II | 52/157 (33.1%) |
| Type III | 37/157 (23.6%) |
| Bovine arch | 27/157 (17.2%) |
| Acute angle type of aortic arch | 48/157 (30.6%) |
| CCA-ICA angle (°), median (IQR) | 148 (136-160) |
| Pre-operative eGFR (mL/min/1.73m2), median (IQR) | 66 (55, 78) |
| Medical history | |
| Hypertension | 123/157 (78.0%) |
| Diabetes mellitus | 72/157 (45.9%) |
| Hyperlipidemia | 117/157 (74.5%) |
| Smoking history | 104/157 (66.2%) |
| Symptomatic stroke within 30 days after CAS | 15/157 (9.5%) |
| Minor stroke | 12/157 (7.6%) |
| Major stroke | 3/157 (1.9%) |
| Presence of postoperative ischemic DWI lesions | 87/157 (55.4%) |
ECST, European Carotid Surgery Trial; CCA, common carotid artery; ICA, internal carotid artery; eGFR, estimated glomerular filtration rate; DWI, diffusion-weighted image; CAS, carotid artery stenting; IQR, interquartile range
| No. | Univariate analysis | ||
|---|---|---|---|
Non stroke 142 |
Symptomatic stroke 15 |
P value | |
| Sex male | 122 (85.9%) | 14 (93.3%) | 0.422 |
| Age (IQR) | 75 (69, 81) | 76 (78, 83) | 0.234 |
| Lt. side | 53 (37.3%) | 10 (66.7%) | 0.028* |
| ECST (IQR) | 74 (65, 82) | 77 (74, 79) | 0.612 |
| Area (IQR) | 85 (78, 90) | 87 (80, 95) | 0.153 |
| Lesion calcification > 1/2 | 30 (21.1%) | 1 (6.7%) | 0.181 |
| Acute angle type of aortic arch | 43 (30.3%) | 5 (33.3%) | 0.807 |
| CCA-ICA angle (IQR) | 148 (136, 160) | 148 (137, 161) | 0.911 |
| Pre-operative eGFR (IQR) | 66 (55, 78) | 67 (54, 75) | 0.974 |
| Medical history | |||
| Hypertension | 110 (77.5%) | 13 (86.7%) | 0.411 |
| Diabetes mellitus | 64 (45.1%) | 8 (53.3%) | 0.541 |
| Hyperlipidemia | 105 (73.9%) | 12 (80.0%) | 0.609 |
| Smoking history | 94 (66.2%) | 10 (66.7%) | 0.971 |
ECST, European Carotid Surgery Trial; CCA, common carotid artery; ICA, internal carotid artery; eGFR, estimated glomerular filtration rate; CAS, carotid artery stenting; IQR, interquartile range
| No. | Univariate analysis | ||
|---|---|---|---|
Negative 70 |
Positive 87 |
P value | |
| Sex male | 59 (84.3%) | 77 (88.5%) | 0.44 |
| Age (IQR) | 73 (67, 79) | 77 (72, 82) | 0.004* |
| Lt. side | 25 (35.7%) | 38 (43.7%) | 0.312 |
| ECST (IQR) | 74 (67, 81) | 75 (63, 82) | 0.835 |
| Area (IQR) | 85 (79, 91) | 85 (78, 90) | 0.622 |
| Lesion calcification > 1/2 | 15 (21.4%) | 16 (18.4%) | 0.635 |
| Acute angle type of aortic arch | 17 (24.3%) | 31 (35.6%) | 0.125 |
| CCA-ICA angle (IQR) | 147 (136, 160) | 149 (137, 160) | 0.971 |
| Pre-operative eGFR (IQR) | 67 (57, 82) | 66 (54, 76) | 0.971 |
| Medical history | |||
| Hypertension | 54 (77.1%) | 69 (73.1%) | 0.743 |
| Diabetes mellitus | 31 (44.3%) | 41 (47.1%) | 0.723 |
| Hyperlipidemia | 53 (75.7%) | 64 (73.6%) | 0.759 |
| Smoking history | 52 (74.3%) | 52 (59.8%) | 0.056 |
ECST, European Carotid Surgery Trial; CCA, common carotid artery; ICA, internal carotid artery; eGFR, estimated glomerular filtration rate; DWI, diffusion-weighted image; IQR, interquartile range
CAS has been reported to be non-inferior to CEA in several studies [3, 7, 8]. However, perioperative minor strokes have been shown to occur more frequently in CAS and are thought to be due to the effect of debris dispersal from carotid stenotic lesions into intracranial vessels during CAS [7, 13]. Memon et al. identified the following four factors that contribute to unfavorable outcomes for CAS: (1) unsuitable aortic arch geometry, (2) steep angulation or bending of the carotid lesion, (3) the shape and nature of the plaque at risk, and (4) the patient's own comorbid disease factors [14]. For fragile and other high-risk plaques, technological innovations such as embolic protection devices and mesh cover stents are expected to reduce the incidence of stroke [15]. The occurrence of perioperative stroke is more pronounced in older adults, and is attributed in part to vascular tortuosity due to progressive atherosclerotic changes [11, 16]. Therefore, it may be important to evaluate and address anatomic risk factors such as aortic arch and ICS for successful CAS.
Anatomical risk factors in the aortic arch
When CAS is performed via the transfemoral approach, the acute angle of aortic arch makes it difficult to control the guiding catheter. There is also a risk of the guiding catheter slipping while the stent is passing through the guiding catheter.
This applies to the bifurcation angle between the aortic arch and brachiocephalic artery in the case of a right-sided ICS and the bifurcation angle between the aortic arch and left CCA in the case of a left-sided ICS. The aortic arch is evaluated into three groups according to the ratio between the length between the brachiocephalic artery bifurcation to the apex of the aortic arch and the diameter of the common carotid artery [12]. In Type III, the angle to the brachiocephalic artery and left CCA is acute, making it difficult to guide a guiding catheter or stent [10, 11, 17]. In the case of bovine arch, where the left CCA branches off from the brachiocephalic artery, the angle from the aortic arch to the left CCA is acute, which is one of the difficulties when treating a left-sided ICS [10, 18]. Difficulty in guiding catheter guidance may necessitate interruption of treatment. Even if guiding is possible, the long time required increases the risk of postoperative thrombotic complications, due to dispersed plaque in the vessel wall and thrombus adhering to the catheter.
In the present study, aortic arch sharpness was not a risk factor for either postoperative symptomatic stroke complications or ischemic variables detected on DWI. At our institution, the optimal guidewire and guiding catheter were selected and used after a thorough preoperative evaluation of the access route, and it is thought that the guiding catheter was guided in a short time and with minimal stress on the vessel wall. If the preoperative CTA or MRA showed a Type III or bovine arch, Simmons-type catheter was used instead of JB2 type for the inner catheter from the beginning. For the guidewire, a softer type with a diameter of 0.035 inch was usually used, but a rigid type was used only when the guiding catheter was passed through the acute angle from the aortic arch to the CCA. Preoperative planning and selection of appropriate inner catheters and guidewires may have contributed to the lack of risk factors even in cases with acute angle type of aortic arch.
Anatomical risk factors in carotid artery stenosis lesions
If the bifurcation angle between CCA and ICA is sharp, the following two surgical steps may be difficult. The first is to cross the lesion with a microguidewire or distal protection device, and the second is to guide the stent into the stenotic lesion. There is an increased risk of postoperative thrombotic complications due to thrombosis and dispersal of lesion plaques caused by difficulty in lesion crossing. Also, if the lesion angulation is severe, it is difficult to deliver the stent on appropriate position.
Sub-analyses of SAPPHIRE and EVA-3S reported a significantly higher incidence of cerebral infarction within 30 days after surgery with sharp bifurcation angles of the CCA to ICA [19, 20]. It was also noted that in two cases where the angle from CCA to ICA was less than 90 degrees, the stent could not be placed as expected [21]. Because closed-cell stents are particularly rigid and inflexible, they may kink or under deploy in vessels with strong bends, making open-cell stents preferable for tortuous lesions [22]. A significant increase in microembolism on postoperative DWI was also reported when close-cell stents were placed in angulated lesions [23].
The study showed that acute CCA-ICA angle was not a risk factor in either postoperative symptomatic stroke complications or ischemic DWI lesions. In all cases, the stents were successfully implanted as planned preoperatively. The use of embolic protection devices is essential to prevent thrombotic complications in patients with lesion crossing difficulties. All patients in this study used a balloon guiding catheter as a proximal embolic protection device, which prevented thrombosis by temporarily blocking blood flow in the CCA during lesion crossing. The filter device is used as a distal embolic protection device to prevent thrombosis after lesion crossing. We believe that the appropriate use of these embolic protection devices contributed to safer CAS for patients with anatomical risks. The use of open-cell stents will have advantages to implant, even in various angulated lesions.
Lesion side and age in carotid artery stenting
In this study, bivariate analysis suggested associations between symptomatic cerebral infarction and left-sided lesions, and between age and DWI lesions.
Zahn et al. reported more complications with CAS for left-sided lesions compared to right-sided lesions [24]. They suggested that the reason for the high complication rate of left-sided carotid stenting, besides anatomic problems, is that the left cerebral hemisphere is often the dominant hemisphere compared to the right. The dominant hemisphere is more clinically sensitive. And we believe that this reason is consistent with the fact that in the present study, although ischemic DWI lesions were not significantly different between left-sided and right-sided CAS, symptomatic stroke was more common on the left side.
Next, similar to the present study, two studies, SPACE [5] and CREST [7], also reported a higher risk of perioperative ischemic stroke in older adults undergoing CAS. This may be due to age-related vascular endothelial damage and atherosclerosis. In this study, we considered the tortuosity of the vascular as an anatomic factor. In addition, severe atherosclerosis is predicted to place a severe load on the vessel when guiding catheters or stents are guided. This would induce endothelial damage, leading to thrombosis and ischemic lesions.
Study limitations
Several limitations should be noted in the present study. First, CEA or medical management alone may be chosen in cases with anatomic high risk from the preoperative radiological estimation. Second, we did not evaluate plaque properties. Third, all patients have been taking oral antiplatelet agents for 2 weeks prior to the procedure, but platelet function prior to CAS was not measured, so the impact of the degree of inhibition of platelet function aggregation on the outcome was not evaluated. Further study will worth to assess the relationship between remaining limitation factors and periprocedural ischemic complication of CAS.
In this study, acute angle type of aortic arch and acute CCA-ICA angle were not associated with symptomatic ischemic stroke within 30 days after CAS and with postoperative ischemic DWI lesions. Even in patients with anatomical factors that make stenting difficult, a safe procedure can be expected by using the appropriate catheter, guidewire, and protection device after a thorough evaluation of vascular anatomy with preoperative imaging.
Ethical approval
This study was approved by Research Ethics Committee of Shimane University School of Medicine (IRB number: 20220112-1). All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. Informed consent was obtained in the opt-out format on the website. Those who declined consent were excluded.
Author contribution
Mizuki KAMBARA: Conceptualization, Methodology, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization.
Yohei SHIBATA: Investigation.
Masahiro UCHIMURA: Investigation.
Fumio NAKAGAWA: Investigation.
Tsutomu YOSHIKANE: Investigation.
Hidemasa NAGAI: Writing - review.
Yasuhiko AKIYAMA: Writing - review & editing, Visualization, Supervision, Project administration.
Acknowledgment
None.
Funding
None.
Conflict of Interest
There are no conflicts of interest.