2013 年 230 巻 1 号 p. 59-66
2013 年 230 巻 1 号 p. 59-66
Cardiovascular disease is common in the patients with end-stage renal disease, who often suffer from secondary hyperparathyroidism (SHP). Vitamin D is considered for the first-line therapy managing SHP in hemodialysis (HD) patients and has a beneficial effect in the chronic inflammation and development of cardiovascular disease. The soluble receptor for advanced glycation end products (sRAGE) may be protective by binding AGE in the pathogenesis of atherosclerotic vascular complications, whereas extracellular RAGE-binding protein (EN-RAGE) represents pro-inflammatory ligands for RAGE. We have hypothesized that vitamin D treatment may alter the levels of sRAGE and EN-RAGE in HD patients. Therefore, this prospective observational study was performed in 51 HD patients with SHP who had low serum 1,25 dihydroxyvitamin D3 (1,25D) levels and elevated intact parathyroid hormone (PTH) levels. We evaluated the changes in the values of sRAGE, EN-RAGE, and other inflammatory marker, interleukin-6 (IL-6), before and at the end of the 8-week calcitriol treatment. After calcitriol treatment, the serum levels of 1,25D were increased, whereas the serum intact PTH levels were decreased. In addition, the sRAGE levels were increased, whereas those of IL-6 were decreased after calcitriol treatment. A positive correlation between 1,25D and sRAGE levels (r = 0.609, P < 0.001) and a negative correlation between sRAGE and EN-RAGE levels (r = −0.368, P = 0.020) were detected after calcitriol treatment. This study suggests that calcitriol treatment could play an anti-inflammatory role through the increasing sRAGE in HD patients with SHP.
Cardiovascular disease (CVD) is more common in the patients with end-stage renal disease (ESRD) than in the general population and a leading cause of morbidity and mortality in patients with ESRD (Foley et al. 1998; Collins 2003; Sarnak et al. 2003). Patients with ESRD also show a chronic inflammatory state, which is associated to vascular calcification (Guerrero et al. 2012). Observational clinical studies support hyperphosphatemia, abnormalities of parathyroid hormone (PTH), and increased vascular calcification as risk factors for CVD among patients with ESRD (Block and Port 2000; Ganesh et al. 2001). ESRD patients with secondary hyperparathyroidism (SHP) have higher levels of serum inflammatory cytokines, which are associated with higher cardiovascular mortality than ESRD patients without SHP (Lu et al. 2006; Tentori et al. 2008). Thus the regulation of systemic vascular inflammation and calcification is an important problem among the hemodialysis (HD) patients. Vitamin D has been considered the first-line therapy to suppress PTH levels for ESRD patients with SHP (Bhan and Thadhani 2009; Patel and Singh 2009).Vitamin D has a wide range of beneficial effects besides PTH suppression, including modulating immune response and cell differentiation (Nagpal et al. 2005; Valdivielso et al. 2009). Because of these effects of vitamin D, it may offer new means to control the inflammatory state of ESRD patients, and vitamin D administration has been shown to improve mortality in HD patients (Teng et al. 2003; Teng et al. 2005).
The receptor for advanced glycation end products (RAGE) is a member of the immunoglobulin superfamily and is expressed at the cell surface of several cell types, including endothelial cells, vascular smooth muscle cells, and cardiomyocytes (Basta 2008). The interaction of the RAGE with AGEs leads to biomedical pathway generating intracellular inflammatory mediators, which could result in further amplification of AGEs generation, vascular inflammation, and subsequent atherosclerosis (Mahajan et al. 2009; Park et al. 2011). The soluble receptor for advanced glycation end products (sRAGE) was known as a decoy receptor by competitively inhibiting the binding of RAGE ligands to RAGE, thereby attenuating the downstream inflammatory responses (Basta et al. 2006; Basta et al. 2010). In experimental models, sRAGE has been shown to attenuate atherosclerosis (Park et al. 1998) and in CKD patients, circulating sRAGE levels are inversely associated with carotid atherosclerosis (Basta et al. 2010). In contrast to sRAGE, extracellular RAGE-binding protein (EN-RAGE), also known as S100A12, acts as pro-inflammatory ligands for RAGE and contributes to occur the atherosclerotic vascular complications (Yang et al. 2001; Mahajan et al. 2009; Nakashima et al. 2010). Mori et al. (2009) showed that EN-RAGE is involved in the acceleration of atherosclerosis in HD patients.
If calcitriol treatment impacts to modulate the inflammatory response, it may be closely correlation with the levels of sRAGE and EN-RAGE. However, until now, the linkage of among the calcitriol, the levels of sRAGE and EN-RAGE has not been investigated, especially in HD patients. Thus, we designed this study to investigate whether parenteral calcitriol therapy affects the levels of sRAGE and EN-RAGE in HD patients with SHP.
This study was a prospective observational design, but we performed a retrospective analysis of patients participating in our previous study (Chang et al. 2012) in which we obtained informed written consents from all participants involved. This investigation was approved by the institutional review board in Gachon Univeristy Gil Medical Center (GCIRB2940-2012).
SubjectsWe screened 289 HD patients visiting our HD unit at Gachon University Gil Medical Center between 2009 and December 2010. Patients were enrolled in the study if they (i) had been on HD for 3 months and were defined as those with serum intact PTH levels greater than 300 pg/ml; (ii) none of the patients had received calcitriol therapy during the previous 1 months or had plasma calcium or phosphorus levels higher than 10.5 mg/dl or 6.0 mg/dl, respectively; (iii) were free of any complicating conditions that could affect serum RAGEs and other inflammatory cytokine levels including chronic inflammatory or autoimmune diseases, with evidence of concurrent infection or malignancy, or on immunosuppressive medications known to interfere with the immune system; and (iv) agreed to participate in the study which was approved by institutional review of board of Gachon University Gil Medical Center. Finally, we analyzed 51 patients (26 males). All HD patients received the conventional 4 hours of dialysis treatment 3 times a week, which consisted of a standard bicarbonate dialysis solution with heparin as an anticoagulant.
Laboratory AnalysisBlood samples were collected for laboratory tests every other month for 1 year from the previous study (Chang et al. 2012). We analyzed the enrolled patients at the beginning and after 8 weeks of calcitriol treatment. After overnight fasting, blood samples to measure serum lipids, creatinine, albumin, calcium and phosphate, hemoglobin, intact PTH, 25 dihydroxyvitamin D3 (25D), 1,25 dihydroxyvitamin D3 (1,25D), and high-sensitivity C-reactive protein (hsCRP) were obtained from all patients. Blood samples were collected and microcentrifuged, and the serum was separated from the blood samples within 1 hour of collection and stored at −70°C until analysis. hsCRP was measured using a high-sensitivity immune-turbidimetric method (ADVIA 2400 Chemistry System, Siemens, Berlin, Germany). The serum intact PTH concentration was measured by chemiluminescence immunoassay (ADVIA Centaur® XP, Siemens, Germany). Serum 25D levels were determined using a chemiluminescence immunoassay (LIAISON® 25 OH Vitamin D TOTAL Assay, DiaSorin Inc., Stillwater, MN, USA), and 1,25D levels were determined using a radioimmunoassay (IDS, 125I RIA Kit, Diasorin Inc., Stillwater, MN, USA). Interleukin-6 (IL-6) was measured with an enzyme linked immunosorbent assay (ELISA) (Human IL-6 Quantikine ELISA Kit, R&D Systems, Inc., Minneapolis, MN, USA). For the quantification of the serum concentrations of sRAGE (Human RAGE Immunoassay; R&D Systems, Minneapolis, USA) and EN-RAGE (CircuLexTM S100A12/EN-RAGE ELISA kit, CycLex Co., Ltd., Nagano, Japan), commercially available ELISA kits were used according to the instructions of the manufacturer.
Calcitriol treatmentIn our HD unit, to suppress PTH levels, initial intravenous calcitriol at 2 µg/ml (Bonky INJ@, Yuyu Pharma, Inc., Seoul, Korea) was administered through an effluent needle at the end of each HD session. Serum calcium and phosphate levels were monitored every 2 weeks during the 8-week calcitriol treatment. The calcitriol dose was maintained as long as the patients did not develop the following conditions: serum PTH levels at or below 300 pg/ml; calcium-phosphate products > 75 mg2/dl2; serum phosphate levels > 7.0 mg/dl; or serum calcium levels > 10.5 mg/dl (Andress 2008).
Statistical analysisContinuous variables were tested for normality with the Kolmogorov-Smirnov test before further statistical analysis. Variables without a normal distribution were either transformed using a natural logarithmic scale and then subjected to parametric tests or analyzed by a non-parametric test. Values with normal distributions are expressed as the mean ± standard deviations, while those without normal distributions are shown as median values and interquartile ranges or geometric mean and 95% confidence interval. Statistical analysis was performed using the t-test for paired values to determine the differences between 2 groups. The correlation between 2 continuous variables was analyzed using a Pearson’s correlation test. All analyses were performed with SPSS (Statistical Package for the Social Sciences) version 15.0 (SPSS Inc., Chicago, IL, USA) or GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA). For all of the statistical analyses, P < 0.05 was considered to be statistically significant.
The baseline characteristics of the patients are presented in Table 1. The mean age was 52.6 ± 14.7 years, and 26 patients were male. The mean duration of dialysis was 59.8 ± 51.7 months, and 43.1% of patients had diabetes mellitus.
HD patients with SHP presented with low serum levels of 1,25D, high intact PTH levels, and elevated inflammatory cytokine levels before calcitriol treatment (Table 2, Fig. 1). Our study showed that the 8-week calcitriol treatment effectively increased serum levels of 1,25D-suppressed intact PTH secretion and decreased inflammatory cytokine levels (IL-6) in HD with SHP patients (Table 2). In addition, calcitriol increased the levels of sRAGE (log sRAGE; 7.75 ± 0.62 pg/ml vs. 8.10 ± 0.54 pg/ml, P < 0.008; Table 2, Fig. 1) but significantly decreased the levels of IL-6 after 8 weeks of treatment (log IL-6; 0.83 ± 0.71 pg/ml vs. 0.52 ± 0.72 pg/ml, P = 0.036); in contrast, hsCRP levels showed no significant changes after calcitriol treatment (0.25 ± 0.70 mg/dl vs. 0.48 ± 1.10 mg/dl, P = 0.207). Surprisingly, calcitriol treatment also increased the levels of EN-RAGE (log EN-RAGE; 5.82 ± 0.82 ng/ml vs. 6.28 ± 0.73 ng/ml, P = 0.012, Table 2, Fig. 1).
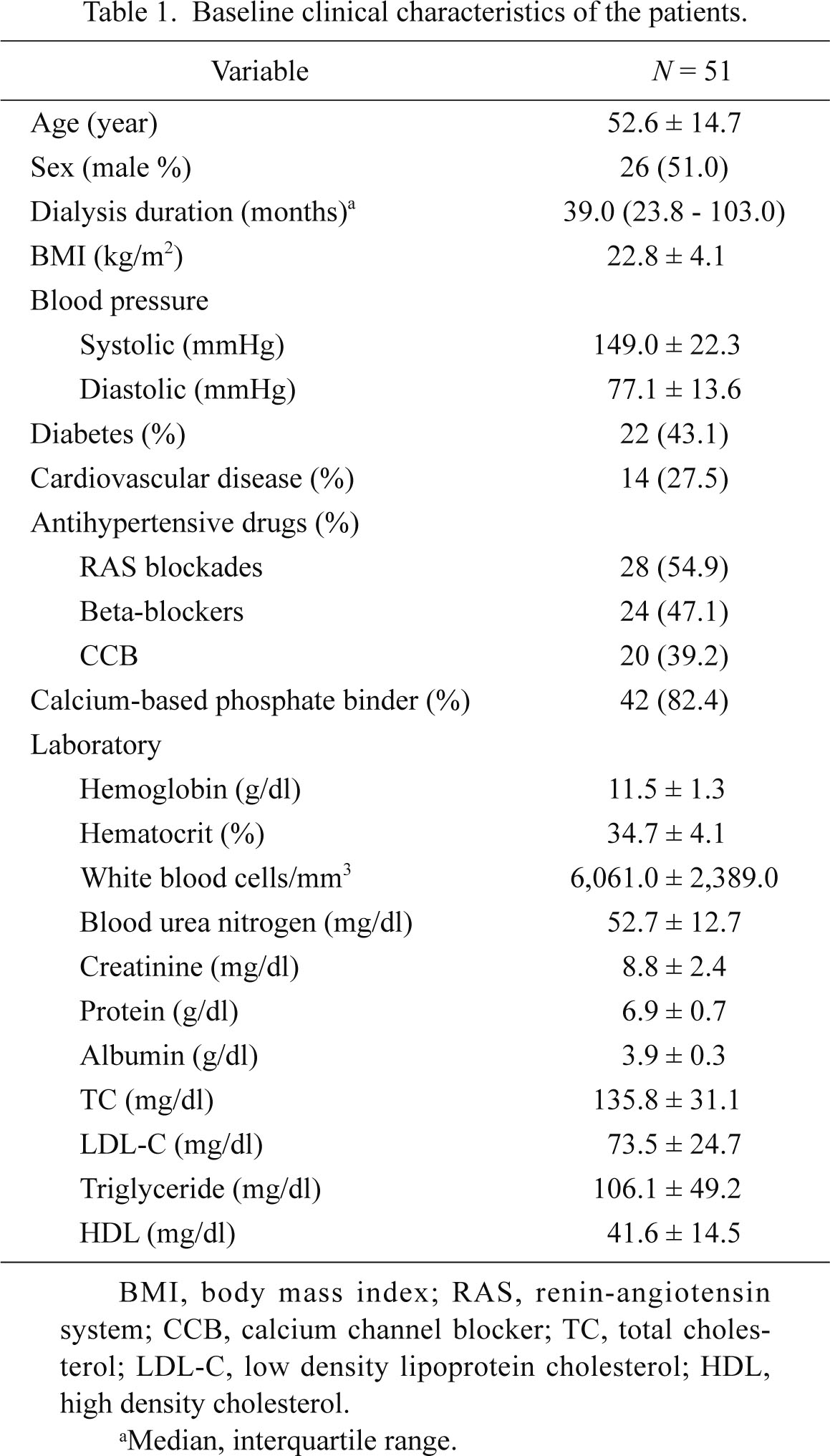
Baseline clinical characteristics of the patients.
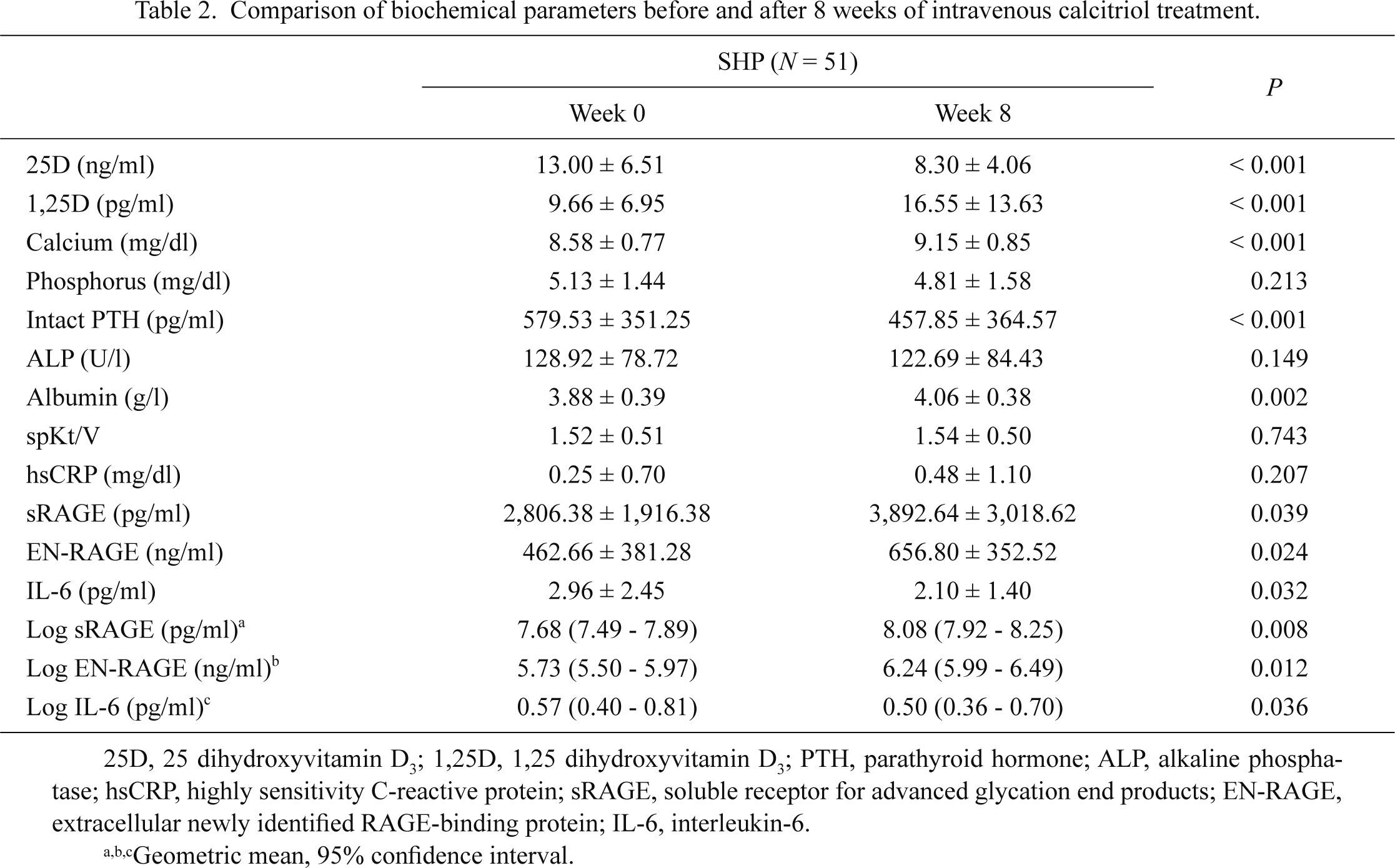
Comparison of biochemical parameters before and after 8 weeks of intravenous calcitriol treatment.
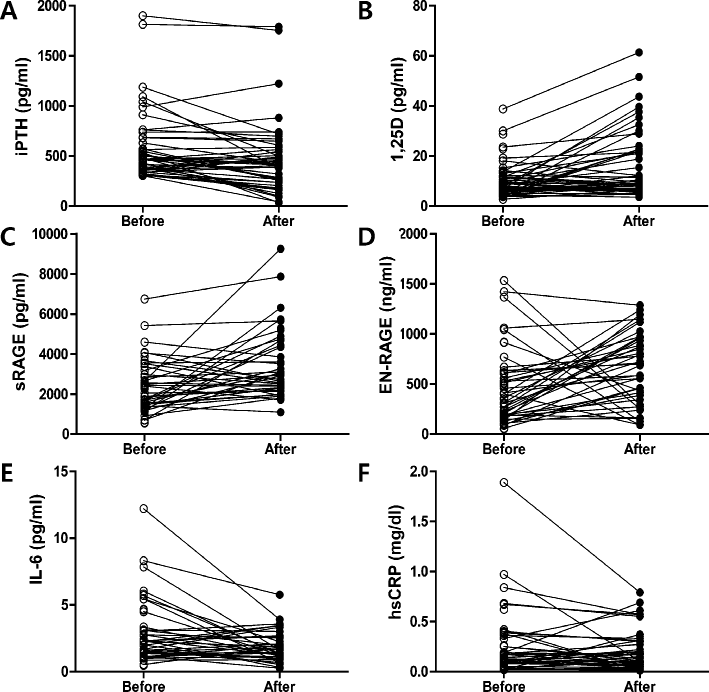
Changes the values before and after calcitriol treatment.
Calcitriol treatment effectively suppressed intact PTH level (A) and increased the levels of 1,25D (B), sRAGE (C), and EN-RAGE (D) (n = 51). It also suppressed IL-6 (E) but not hsCRP levels (F) (n = 51).
The relationship between 1,25D and sRAGE was investigated in SHP patients receiving calcitriol treatment. We found that there was a positive correlation between the levels of 1,25D and sRAGE (r = 0.609, P < 0.001) and a negative correlation between the levels of sRAGE and EN-RAGE (r = −0.368, P = 0.020) after calcitriol treatment (Fig. 2).
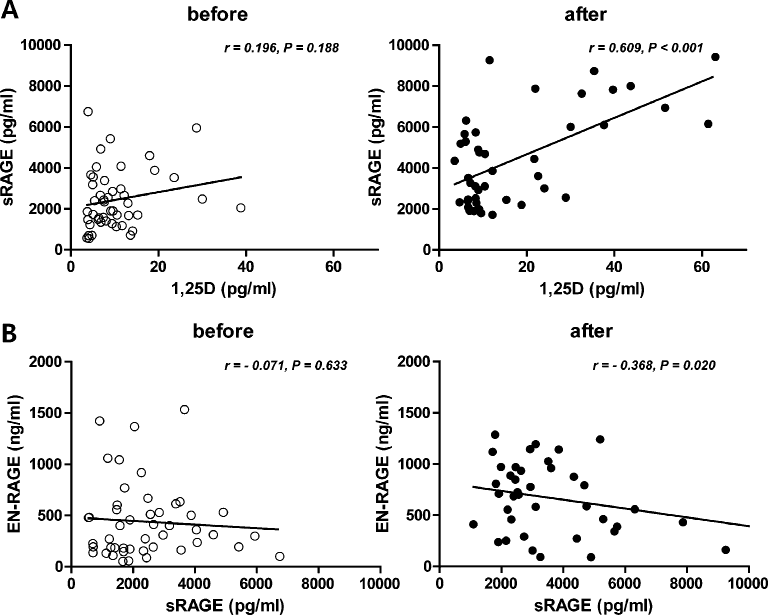
Correlations between the values before and after calcitriol treatment.
Before calcitriol treatment, there are no association between the values; however, a positive correlation between 1,25D and sRAGE levels (A) and a negative correlation between sRAGE and EN-RAGE levels (B) were detected after calcitriol treatment (n = 51).
This study found that the levels of sRAGE were significantly increased, whereas those of IL-6 were decreased, after calcitriol treatment in HD patients with SHP. Unexpectedly, calcitriol treatment also increased the concentration of EN-RAGE.
Calcitriol has been used for SHP in ESRD patients to reduce the serum levels of intact PTH, and the current study showed that calcitriol treatment effectively suppressed the intact PTH levels while increasing the 1,25D levels. Moreover, because vitamin D has beneficial effects besides intact PTH suppression, we sought to investigate the influence of calcitriol on inflammatory marker levels, such as hsCRP, IL-6, sRAGE, and EN-RAGE, in HD patients with SHP. These patients manifested higher levels of serum inflammatory cytokines and oxidative stress, which are associated with higher all-cause and cardiovascular-specific mortality compared to non-SHP patients (Lu et al. 2006; Tentori et al. 2008). Vitamin D deficiency and SHP are thought to promote CVD in ESRD patients (Bucala and Cerami 1992; Block et al. 2004), in addition to the traditional risk factors for CVD. Moreover, atherosclerosis has recently been characterized as an inflammatory disease (Ross 1999). Because CVDs are the leading causes of high mortality in patients with ESRD (Foley et al. 1998), the protective effect of 1,25D administration on CVD manifestations in HD patients has been hypothesized and demonstrated in previous studies (Park et al. 1999; Shoji et al. 2004).
AGEs, in combination with their receptors (RAGE), have been emphasized as factors associated with atherosclerosis and may play an important role in the development of CVD in patients with CKD (Sarnak et al. 2003; Kendrick and Chonchol 2008; Stenvinkel et al. 2008). RAGE is involved in many pathological states, such as vascular disease, diabetic complications, and inflammatory diseases (Wautier et al. 1996; Schmidt et al. 2001; Yonekura et al. 2005). In particular, sRAGE acts as a decoy receptor to inhibit the AGE-RAGE interaction and has protective effects on various organ systems (Yonekura et al. 2003; Koyama et al. 2007). One example of the protective role of sRAGE was presented by an observational study showing that the plasma concentration of sRAGE is significantly lower in patients with coronary artery disease than in age-matched healthy controls (Falcone et al. 2005) and that carotid atherosclerosis is inversely associated with the sRAGE level in diabetic and non-diabetic patients (Cipollone et al. 2003; Koyama et al. 2005). Furthermore, sRAGE is an inverse marker of left ventricle hypertrophy in CKD patients (Leonardis et al. 2012).
In this study, sRAGE levels were increased, whereas IL-6 levels were decreased, after 8 weeks of calcitriol treatment. In vitro studies have shown that calcitriol has pro-inflammatory properties and can weaken the stimulating effects of RAGE and IL-6 (Kendrick and Chonchol 2008; Stenvinkel et al. 2008; Talmor et al. 2008; Talmor-Barkan et al. 2011). The effects of calcitriol on RAGE mRNA expression have also been examined in human umbilical vein cord endothelial cells in a previous study (Talmor-Barkan et al. 2011), which found that RAGE mRNA expression was enhanced in the presence of low calcium or PTH and was decreased in the presence of calcitriol, which inhibited the expression of pro-inflammatory and pro-atherosclerotic parameters including RAGE and IL-6. In addition, this study provided evidence that calcitriol may regulate these actions by affecting the activity of the NFκB and p38 MAPK pathways (Talmor et al. 2008).
In contrast to sRAGE, S100A12, also known as EN-RAGE, triggers the RAGE pathways as a proinflammatory ligand, regulating monocyte migration and inducing proinflammatory cytokine production in macrophages (Hofmann et al. 1999; Yang et al. 2001; Yang et al. 2007). Elevated plasma EN-RAGE concentrations have been positively associated with carotid artery atherosclerosis (Mori et al. 2009; Kim et al. 2012), and Nakashima et al. showed that EN-RAGE concentration was a direct predictor of all-cause and CVD-related mortality, even after adjusting for confounders (Nakashima et al. 2010). However, we did not find a reduction in EN-RAGE levels after calcitriol treatment; in fact, we observed an increase. The sRAGE levels we detected were similar to those in previous reports (Kalousova et al. 2006; Nakashima et al. 2010), although our EN-RAGE levels were relatively higher than those previously reported (Mori et al. 2009; Nakashima et al. 2010; Shiotsu et al. 2011; Zakiyanov et al. 2011). The precise reason for such discrepancy is not clear. However, a variety of different factors can affect level of sRAGE and EN-RAGE. These differences may be due to the small number of patients enrolled in this study and/or differences in patient populations that already showed much more pro-inflammatory conditions with SHP. In addition, our subjects had relatively long dialysis durations; therefore, higher levels of EN-RAGE would be expected in this population. However, the levels of sRAGE and EN-RAGE were similar to those of previous reports on ESRD patients in Korea (Kim et al. 2012).
We also hypothesized that SHP status would affect EN-RAGE levels, as the positive relationship between 1,25D and sRAGE and the inverse relationship between sRAGE and EN-RAGE were discovered after calcitriol treatment, although this warrants further investigation. Because the observed increase in sRAGE and reduction in EN-RAGE may be related to CVD, hypertension, and atherosclerosis in patients with ESRD (Mori et al. 2009; Nakashima et al. 2010; Shiotsu et al. 2011; Wu et al. 2011; Kim et al. 2012), the previously unreported relationship between SHP and EN-RAGE levels merits additional study.
Our study has several limitations. First, this study was conducted on limited number of subjects and over a relatively short period of observation, and we could therefore not observe the effect of calcitriol on long-term cardiovascular mortality. Thus, follow-up data would be required to clarify the effect of calcitriol on the expression of inflammatory markers, sRAGE, and EN-RAGE. Second, this study was a prospective observational study, but the analyses were done retrospectively. Despite these limitations, this is the first report to provide evidence that sRAGE levels are increased after calcitriol treatment in HD patients with SHP.
To summarize, we observed that the levels of sRAGE were significantly increased, while those of IL-6 were decreased, after calcitriol treatment in HD patients with SHP. However, calcitriol treatment unexpectedly increased the concentration of EN-RAGE. Our findings suggest that calcitriol treatment could play an anti-inflammatory role through the sRAGE but not EN-RAGE in HD patients with SHP. Larger-scale and prospective studies over longer periods of time are warranted to investigate whether the administration of calcitriol could ameliorate CVD via alterations in RAGE proteins.
This work was supported by the Gachon University research fund of 2012 (GCU-2012-M082).
The authors declare no conflict of interest.