2013 年 230 巻 2 号 p. 75-82
2013 年 230 巻 2 号 p. 75-82
Poorly differentiated thyroid carcinoma (PDTC) is a newly recognized histological type of malignant thyroid tumor, accounting for about 2 - 13% of all thyroid carcinomas. PDTC is considered as a morphologically and biologically intermediate stage between well-differentiated thyroid carcinoma and anaplastic thyroid carcinoma. PDTC preferentially manifests bone metastases. We here established a cell line from a resected tumor specimen from a 70-year-old male patient with PDTC who presented with multiple bone metastases. This new thyroid tumor cell line was designated as DH-14-3 and was subsequently grown in culture for several years. DH-14-3 cells express thyroglobulin in the cytoplasm and thyroid transcription factor-1 in the nuclei, both proteins of which are specific markers for the thyroid gland. Importantly, triiodothyronine (T3) was detected in the cultured medium of DH-14-3 cells, in which, however, thyroxine (T4) was undetectable. Moreover, DH-14-3 cells secreted interleukin-8, transforming growth factor-β1, vascular endothelial growth factor, matrix metalloproteinase-1 and parathyroid hormone-related protein, all of which may be responsible for the aggressiveness or bone metastasis of PDTC. Thus, the production of these proteins may reflect the metastatic potential of this cell line. DH-14-3 cells also express CXC chemokine receptor-4 and epidermal growth factor receptor, and carry a missense mutation in the p53 tumor suppressor gene. In fact, transplantation of DH-14-3 cells into the back of nude mice resulted in the formation of tumors, thereby confirming the capability of tumorigenesis. DH-14-3 cells may be useful for investigating the biological features of PDTC and will contribute to the therapeutic study of thyroid cancer.
In 1983, Sakamoto and coworkers proposed the entity of poorly differentiated carcinoma of the thyroid (Sakamoto et al. 1983). The presence of non-glandular components with the cancer cells showing solid, trabecular, and scirrhous patterns was a criterion for this condition. A solid pattern is a sheet-like arrangement, while a trabecular pattern is a cord-like arrangement. Insular thyroid carcinoma is the best characterized poorly differentiated thyroid carcinoma (PDTC), with large well-defined nests of tumor cells that are often sharply separated by created clefts (Carcangiu et al. 1984). Immunohistochemical staining has recently become an invaluable tool in diagnosing and understanding the pathophysiology of thyroid tumors. PDTCs express thyroglobulin, which distinguishes them from anaplastic thyroid carcinoma (ATC) that does not express thyroglobulin. PDTCs also express thyroid transcription factor-1 (TTF-1), a specific marker of thyroid carcinoma and lung carcinoma (Nakamura et al. 2002; Patel and Shaha 2006). PDTCs are negative for calcitonin, chromogranin, and carcinoembryonic antigen (Patel and Shaha 2006). PDTCs account for about 2-13% of all thyroid carcinomas (Sakamoto et al. 1983; Lam et al. 2000; Pellegriti et al. 2002; Volante et al. 2004; Lin et al. 2007). PDTC is a morphologically and biologically intermediate stage between well-differentiated thyroid carcinoma (WDTC) and ATC. PDTC is also intermediate stage between WDTC and ATC in terms of prognosis and frequency of distant metastasis. For PDTC patients, previous studies have reported 5, 10, and 15-year survival rates 46-85%, 34-67%, and 0%, respectively (Sakamoto et al. 1983; Lam et al. 2000; Volante et al. 2004). Distant metastases were observed in approximately 50% of all PDTC patients (Pellegriti et al. 2002; Wreesmann et al. 2002). No significant difference was observed between PDTC and other thyroid cancers in regard to the site of metastasis, and the most common site of metastasis is the lung, followed by the bones.
Recent studies have demonstrated that chemokine receptors are expressed on a large number of human cancer cells (Sancho et al. 2006). Some reports have shown that chemokine receptor interactions are involved in proliferation, migration, invasion, and the determination of the metastasis site of tumor cells. For example, tumor cells that up-regulate CXC chemokine receptor-4 (CXCR4) frequently metastasize to organs that express high levels of CXCL12, the ligand of CXCR4, such as the bone marrow, lungs, and lymph nodes (Muller et al. 2001). The CXCR4-CXCL12 axis also mediates breast cancer metastasis to the lung and lymph nodes (Muller et al. 2001) as well as prostate cancer and neuroblastoma metastasis to the bone (Taichman et al. 2002). The CCR-7-CCL21 interaction plays a central role in lymphocyte trafficking and homing to lymph nodes (Zhang et al. 2007). On the basis of these findings, recent studies reported that CCR-7 positive tumor cells preferentially metastasize to lymph nodes. CCR7 is involved in lymph node metastasis of esophageal squamous cell carcinomas (Ding et al. 2003), non-small cell lung carcinomas (Takanami 2003), gastric carcinomas (Mashino et al. 2002), malignant melanomas (Wiley et al. 2001), and thyroid carcinomas (Sancho et al. 2006).
Cytokines and some proteins play an important role in the dynamics of cancer metastasis and aggressiveness. IL-8 and VEGF are angiogenic and mitogenic factors, and are therefore involved in tumor progression and metastasis (Bergers and Benjamin 2003; Bendre et al. 2005). Matrix metalloproteinases (MMPs) are proteolytic enzymes that degrade extracellular matrix components and play a crucial role in tumor invasion and metastasis (Rundhaug 2003). TGF-β1 functions as both a tumor suppressor and a tumor promoter, however it acts more like a tumor promoter in advanced tumorigenesis through the alteration of TGF-β1 responsiveness, epithelial mesenchymal transition, and immune suppression (Massague et al. 2000).
Only a few established cell lines of PDTC have been reported (Asakawa and Kobayashi 1999; Rodrigues et al. 2007), but the biological features of PDTC remain to be investigated. We herein report the establishment and characteristics of a new PDTC cell line, DH-14-3. We also investigated whether DH-14-3 cells possessed biological properties involved in aggressiveness and metastasis.
A patient with PDTCA 70-year-old Japanese male was admitted because of a goiter presented with back pain, right coxalgia, and left gonalgia. PDTC was suspected from fine-needle aspiration biopsy prior to operation. Both thyroid tumor and bone metastases were successfully detected by single-photon emission computer tomography with 67Ga-citrate. Bone metastases were also assessed by whole-body bone scintigraphy (with 99mTc MDP) and magnetic resonance imaging. Bone metastases were present in the left iliac bone, right pubic bone, fourth cervical vertebra, and first and seventh thoracic vertebrae. Blood examination showed a marked elevation of serum thyroglobulin at 15,000 ng/ml (normal range, 0-32.7 ng/ml in our constitution), and a slight elevation of thyroid stimulating hormone (TSH) at 6.18 μIU/ml (normal range, 0.31-4.69 μIU/ml). The serum concentration of free T4 was 1.07 ng/ml (normal range, 0.71-1.85 ng/ml), and free T3 was 3.24 pg/ml (normal range, 2.5-4.3 pg/ml). Thus, thyroid function was within the normal range. Serum carcinoembryonic antigen (CEA) was slightly elevated (6.6 ng/ml; normal range, 0-5.0 ng/ml), but calcitonin was within the normal range (18 pg/ml; normal range, 15-86 pg/ml).
Total thyroidectomy and radical neck dissection were performed. The tumor had invaded the adjacent fat and muscle tissue and metastasized to the peripheral lymph nodes. On histological examination, the tumor displayed an insular pattern (Fig. 1) with abundant vascular formation and some solid and nesting pattern. Nuclear grooves and intranuclear bodies that are characteristic of papillary thyroid carcinoma were also observed. This tumor tested positive for thyroglobulin (Fig. 2A) and TTF-1 (Fig. 2B), negative for CEA (Fig. 2C), calcitonin (Fig. 2D), and chromogranin A (Fig. 2E). On the basis of these histological and immunohistological findings, we definitively diagnosed this patient with PDTC. With rapid deterioration, the patient died 3 months after operation. Lung metastasis and hypercalcemia were not detected; therefore the cause of death was considered to be cancer cachexia. We obtained informed consent from the patient to use the specimen for our research.
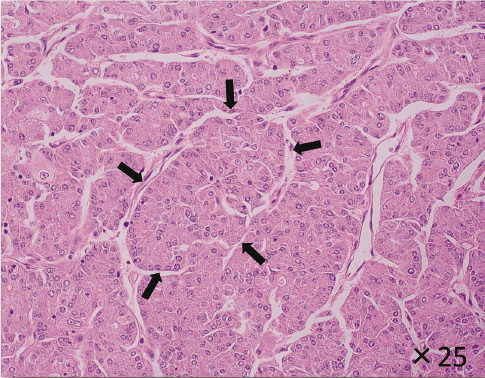
The appearance of the patient's thyroid tumor (Hematoxylin-eosin staining).
Thyroid tumor of this patient displayed an insular pattern (indicated by black allows) with abundant vascular formation under light microscope.

Immunohistochemical staining of this patient’s thyroid tumor.
Immunohistochemical staining of this thyroid tumor tested positive for thyroglobulin (A) and TTF-1 (B), and negative for carcinoembryonic antigen (C), calcitonin (D), and chromogranin A (E).
Williams’ Medium E (with L-glutamine, without sodium bicarbonate) was purchased from SIGMA-ALDRICH (St. Louis, NJ). Monoclonal anti-human CXCR4 (fusin) antibody was purchased from R&D system (Minneapolis, MN). The human IL-8 and VEGF immunoassay kits were purchased from BIOSOURCE (San Jose, CA). Matrix Metalloproteinase-1 (MMP-1) Human Biotrak ELISA System was purchased from GE Healthcare UK Limited (Little Chalfont, UK).
Isolation and establishment of the cell lineA tissue sample was obtained immediately after tumor resection. The sample was minced with a scalpel and then incubated with 2,000 U/ml dispase in RPMI medium at 37°C for 30 min. After incubation, the sample was rinsed in phosphate-buffered saline (PBS) and centrifuged at 1,200 rpm for 5 min. The cell pellet was resuspended in Williams’ Medium E and subsequently incubated at 37°C in 10% CO2. We developed a subculture of these cells, which we called DH. Cloning of the DH cell line was achieved by limiting dilution. Primary clones were classified into 20 groups. VEGF and parathyroid hormone-related protein (PTHrP) concentrations were highest in the culture supernatant of clone number 14 (DH-14); therefore DH-14 cells were re-cloned into 12 groups by limiting dilution. Among these cell lines, DH-14-3 produced the highest VEGF and PTHrP concentrations in the culture supernatant. We used the DH-14-3 in all of the experiments presented.
Cell cultureDH-14-3 cells were cultured in Williams’ Medium E supplemented with 10% fetal calf serum (Sigma-Aldrich), 2.2 g/l sodium bicarbonate, 100 U/ml penicillin (Meiji Seika) and 100 μg/ml streptomycin (Meiji Seika). The cells were grown in a humidified incubator at 37°C in 10% CO2. The medium was replaced every 3 days, and DH-14-3 cells have now been stably cultured for more than 3 years.
Cell growthTo determine the growth of DH-14-3, the cells were seeded in a 10-cm culture dish at 1 × 105 cells/ml in triplicate and incubated for 7 days. The cells were counted daily using a hemocytometer in triplicate. The doubling time was approximately determined from an exponential curve.
Chromosomal analysisA chromosomal analysis was performed by the G-banding method, which was entrusted to Nihon Gene Research Laboratories Inc. (Sendai, Japan).
Analysis of p53, K-ras, and BRAF gene mutationsAnalysis of p53 and k-ras mutations was entrusted to Mitsubishi Chemical Medience Corporation (Tokyo, Japan). p53 gene mutation was analyzed in exons 5, 6, 7, 8, and 9. k-Ras gene mutation was analyzed in codons 12 and 13. Analysis of BRAF gene mutation was entrusted to Nihon Gene Research Laboratories Inc. (Sendai, Japan). BRAF gene mutation was analyzed in exon 15. Genomic DNA was extracted from cultured DH-14-3 cells, and specific genes were amplified by PCR. Gene alterations were detected by direct sequencing of the PCR products and by comparison with Genbank sequences.
XenotransplantationThe cells (1 × 106) suspended in 1 ml of Williams’ Medium E were transplanted subcutaneously into the backs of nude mice. The mice were sacrificed 4 weeks after transplantation, and the tumors were examined histologically by hematoxylin-eosin staining and immunohistochemical staining.
Immunocytochemical stainingFor detection of CXCR4 and CCR7 in cultured cells, cells were seeded in the Lab-Tek II Chamber Slide (Nalge Nunc International, Tokyo, Japan) and grown overnight in a humidified incubator at 37°C. Monolayer cells were fixed with 2% paraformaldehyde for 15 min, and then soaked in 3% H2O2 in methanol for 15 min to block endogenous peroxidase activity. Mouse monoclonal anti-human CXCR4 (fusin) (R&D system) and CCR7 antibodies (R&D system) were applied for 2 h at 37°C. The primary antibody was visualized using a Histofine Simple Stain MAX-PO kit (Nichirei, Tokyo, Japan), according to the manufacture’s instruction.
For thyroglobulin and TTF-1 detection in cultured cells, DH-14-3 cells were released from their substrate by trypsinization, and a cell block was prepared by centrifugation. This cell block was fixed with 10% formalin, embedded in paraffin, and then sectioned on a microscope slide. After deparaffinization, the sections were rehydrated and immersed in 3% H2O2 in methanol for 15 min. The sections were then autoclave for antigen retrieval for 10 min at 110°C. Antibodies for thyroglobulin (DAKO, Tokyo, Japan) and TTF-1 (DAKO) were applied and incubated overnight at 4°C. The expression levels were determined using the peroxidase-labeled streptoavidin-biotin complex, according to the manufacturer’s instructions.
Measurement of proteins in culture supernatantsFor measurement of thyroid hormones (T3, T4), thyroglobulin, cytokines, and growth factors in the culture supernatants, 1 × 106 DH-14-3 cells were seeded in a 10-mm tissue culture dish in 10 ml of medium. We changed the medium when the cells were sub-confluent (approximately 2-3 × 106 cells/dish); the supernatants were collected after 3 days, centrifuged at 1,500 rpm for 5 min, and stored at −80°C until use.
Measurement of thyroglobulin, T3, T4, PTHrP, and TGF-β1 was entrusted to Mitsubishi Kagaku Bio-Clinical Labs, Inc. (Tokyo, Japan). T3 and T4 were measured using a chemiluminescent immunoassay, PTHrP was measured using an immunoradiometric assay, and TGF-β1 was measured using an enzyme immunoassay. The concentrations of IL-8, MMP-1 and VEGF in the culture supernatants were measured with ELISA. The detection range for IL-8 was 15.6-1,000 pg/ml, MMP-1 was 6.25-100 ng/ml, and VEGF was 23.4-1,500 pg/ml. The IL-8 and VEGF concentrations were over the detection range of the ELISA kit; therefore the culture supernatants were appropriately diluted.
Reverse-transcription and quantitative real-time PCRDH-14-3 cells were released from their substrate by trypsinization, washed twice with PBS, and centrifuged at 1,500 rpm for 5 min. The ISOGEN reagent (Nippon Gene, Tokyo, Japan) was added to the cell precipitates, and RNA was isolated from the lysate by chloroform extraction and isopropyl alcohol precipitation. Total RNA content and purity was estimated at 260 and 280 nm. Reverse transcription of the RNA samples was performed using Moloney murine leukemia virus reverse transcriptase and the Oligo (dT) 20/P7 primer provided in the first-strand cDNA synthesis Kit (ReverTraAce-α- TM., TOYOBO CO. LTD., Osaka, Japan). The reaction mixture was incubated at 30°C for 10 min, at 42°C for 20 min, at 99°C for 5 min to inactivate reverse transcriptase. The mixture was subsequently cooled at 4°C for 5 min.
Quantitative real-time PCR was performed using Light Cycler (Roche Diagnostics, Tokyo, Japan) and SYBR Premix Ex TaqTM (Perfect Real Time; Takara Bio Inc., Otsu, Japan) according to the manufacturer’s instructions. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a reference for quantifications. Shuttle PCR was run, and the protocol involved denaturing (95°C for 10 s; followed by 95°C for 5 s, and 66°C for 20 s for 45 cycles; and melting 95°C for 0 s, 65°C for 15 s, and 95°C for 10 s). The mixture was subsequently cooled at 4°C. The sequences of the specific primer used in this study are shown below.
GAPDH
forward: 5′ - GCACCGTCAAGGCTGAGAAC - 3′
reverse: 5′ - TGGTGAAGACGCCAGTGGA - 3′
IL-8
forward: 5′ - ACACTGCGCCAACACAGAAATTA - 3′
reverse: 5′ - TTTGCTTGAAGTTTCACTGGCATC - 3′
MMP-1
forward: 5′ - ACAACTGCCAAATGGGCTTGA - 3′
reverse: 5′ - CTGTCCCTGAACAGCCCAGTACTTA - 3′
NIS (Sodium Iodide Symporer)
forward: 5′ - GACTGACACCCTGGGATGGAA - 3′
reverse: 5′ - AAGGCAATCCAGCCAATCAGAG - 3′
PTHrP
forward: 5′ - CCATCTGATCGCAGAAATCCAC - 3′
reverse: 5′ - AGGTATCTGCCCTCATCATCAGAC - 3′
TGF-β1
forward: 5′ - AGCGACTCGCCAGAGTGGTTA - 3′
reverse: 5′ - GCAGTGTGTTATCCCTGCTGTCA - 3′
Thyroglobulin
forward: 5′ - CTGGAGCACTCCACGGATGA - 3′
reverse: 5′ - AGGCACTGGCCATGTCGATTA - 3′
TPO (thyroid peroxidase)
forward: 5′- CAGCTCACTTGCACCCAGGA - 3′
reverse: 5′ - ACCGTCTGCACACTCGTTCAC - 3′
TSHR (thyroid-stimulating hormone receptor)
forward: 5′ - GCAAGATTTCAGCTTATGTGGCCTA - 3′
reverse: 5′ - ATTGAGTGTGCTCTGCCCATAATTC - 3
TTF-1
forward: 5′ - TCCGTGGCCCTCAAGTTCTC - 3′
reverse: 5′ - AGCCTTGATTAGTTTCCGGGTTTC - 3
VEGF
forward: 5′ - TGTCCTCACACCATTGAAACCACTA - 3′
reverse: 5′ - AATGGGCACGTGGATCCTG - 3′
The PCR products were analyzed using 2.5% agarose gel containing 0.05 μg/ml ethidium bromide and visualized under UV light.
Phase-contrast light microscopy of DH-14-3 cells is shown in Fig. 3A. The DH-14-3 cells grew in a monolayer and showed a pavement-like pattern. The cells were round or polygonal. Immunocytochemical staining with CXCR4 and CCR7 specific antibodies showed the presence of CXCR4 (Fig. 3B) and CCR7 (Fig. 3C) proteins, respectively, in the cell membrane. Thyroglobulin (Fig. 3D) and TTF-1 (Fig. 3E) were also present in the cells. Thyroglobulin immunoreactivity was observed in the cytoplasm, and TTF-1 was found in the nuclei. These results are consistent with the fact that DH-14-3 cells originated from the thyroid gland. These cells have retained the characteristics of thyroid tissue after many subcultures.
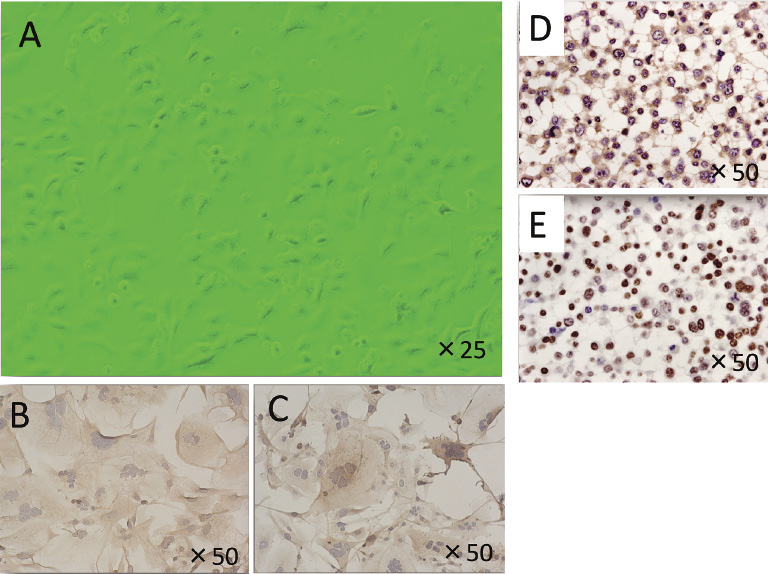
Phase-contrast microscopic appearance and immunocytochemical staining of DH-14-3 cells.
Phase-contrast microscopic appearance of sub-confluent DH-14-3 (A). The DH-14-3 cells grew in a monolayer and partially showed a pavement–like pattern. The cells were round or polygonal. Immunocytochemical staining with CXCR4 and CCR7 specific antibodies showed the expression of CXCR4 (B) and CCR7 (C) proteins. Thyroglobulin immune-reactivity was observed in the cytoplasm (D), and TTF-1 was found in the nuclei (E).
Transplantation of DH-14-3 cells into the back of nude mice resulted in the formation of tumors. Thus, the tumorigenesis of DH-14-3 cells was confirmed by xenograft transplantation experiments. The xenograft tumor displayed a solid pattern with trabecular interstitial tissues, large vesicular nuclei or prominent nucleoli, and eosinophilic cytoplasm (Fig. 4). These are characteristics of PDTC, but differ from the primary tumor.
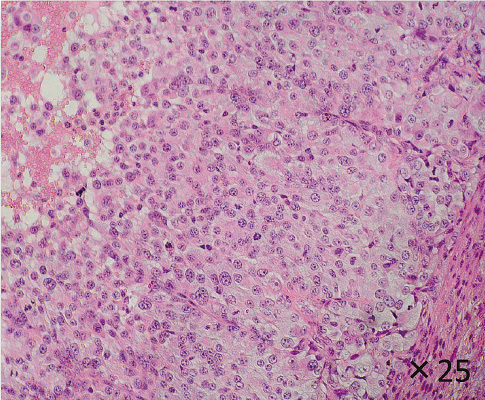
The appearance of the xenograft tumor (Hematoxylin-eosin staining).
The xenograft tumor of DH-14-3 into the nude mice displayed mainly a solid pattern with trabecular interstitial tissues. These morphological appearances were consistent with characteristic of PDTC, but differ from the primary tumor.
The growth curve of the DH-14-3 cells is shown in Fig. 5. DH-14-3 cells grew exponentially for 1 week. The doubling time was approximately 1.7 days, and the plating efficiency was approximately 80%.

Cell growth curve of DH-14-3 cells.
DH-14-3 cells grew exponentially for 1 week. The doubling time was approximately 1.7 days, and the plating efficiency was approximately 80%.
Chromosomal analysis was performed by the G-banding method on twenty cells (not shown). A large number of deletions, translocations, and insertions were detected. The number of chromosome ranged from 45 to 51, and 94 to 112. DH-14-3 cells have two karyotypes, diploidy and tetraploidy. The X-chromosome and Y-chromosome were observed, which is consistent with sex of this patient.
Analysis of p53, K-ras and BRAF gene mutationsWe analyzed p53 for mutational hotspots, exons 5, 6, 7, 8, and 9. We detected a mutation in codon 273, TGT (arginine) to CGT (cytosine) transition. However, no alteration was detected in k-ras or BRAF gene.
Measurement of thyroid hormones, thyroglobulin, and cytokines in the culture supernatantsTo determine whether the DH-14-3 cells originated from thyroid epithelial cells, we measured thyroglobulin, T3, and T4 in the culture supernatants. T3 was detectable (20 ng/dl), but T4 was below the limit of detection. We also determined levels of IL-8, VEGF, TGF-β1, MMP-1, and PTHrP in the culture supernatants. The concentration of each factor is shown in Table 1.

The concentrations of several cytokines and enzymes in the culture supernatants.
DH-14-3 produced and secreted several cytokines and enzymes into the culture media. Production of thyroglobulin and T3 indicate that DH-14-3 cells retain original properties of the thyroid grand. IL-8 and VEGF concentrations were especially high.
We determined whether mRNAs for proteins specific for the thyroid gland were expressed in the DH-14-3 cells, including thyroglobulin, thyroid peroxidase (TPO), TSHR, TTF-1, and NIS. The expression of these mRNAs, except for TPO mRNA, was detected by RT-PCR, and the PCR products were confirmed by electrophoresis (Fig. 6A). Likewise, the expression of IL-8, VEGF, TGF-β1, MMP-1, and PTHrP mRNAs was detected by RT-PCR (Fig. 6B). The DH-14-3 cells express mRNAs of the aforementioned cytokines and growth factors.
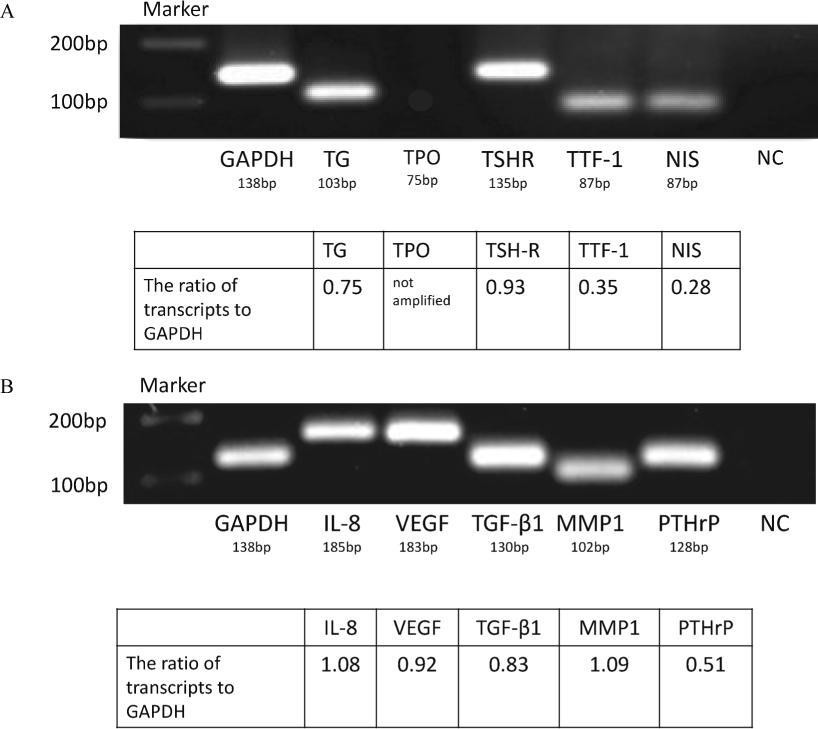
Electrophoresis of the PCR products.
We analyzed the expression of mRNAs for thyroid gland-specific proteins in DH-14-3 cells, including thyroglobulin (TG), thyroid peroxidase (TPO), thyroid-stimulating hormone receptor (TSHR), thyroid transcription factor-1 (TTF-1), and sodium iodine symporter (NIS). The expression of these protein mRNAs, except for TPO mRNA, was detected by RT-PCR, and the PCR products were confirmed by electrophoresis (A). NC stands for negative control. RT-PCR also revealed the expression of IL-8, VEGF, TGF-β1, MMP-1 and PTHrP mRNAs (B).
We describe here the establishment and characteristics of a new PDTC cell line, DH-14-3, and demonstrate its potential as a laboratory model for human thyroid carcinoma in vitro. The DH-14-3 line established from a human thyroid tumor can be propagated by routine culture methods and displays excellent cloning efficiency. The identity of DH-14-3 cell line originated from a human thyroid tumor is provided by its morphology and immunohistochemical characterization. The histological examination of the original tumor displayed mainly insular patterns (with some solid and nesting patterns) with abundant vascularization, which was consistent with the established properties of PDTC histologically. Xenograft tumors of DH-14-3 in nude mice displayed solid and nesting patterns, and not an insular pattern. Although the xenograft tumor did not resemble original tumor, it had characteristics of PDTC.
Thyroid-specific proteins such as thyroglobulin and TTF-1 were present in DH-14-3 cells, but CEA and calcitonin were not expressed. DH-14-3 cells secreted thyroglobulin and thyroid hormone T3 to the culture medium, but not T4. In vivo T4 is metabolized by deiondinase to become T3; therefore T4 concentration is normally higher than T3 concentration. We did not measure the deiodinase activity of DH-14-3 cells; there may be possibility that the deiodinase activity is increased in DH-14-3 cells. In this context, iodine may be derived in part from the fetal calf serum, because the medium did not contain iodides.
DH-14-3 cells also have chromosomal abnormalities, such as deletions, translocations, and insertions. DH-14-3 cells have two karyotypes, diploidy and tetraploidy. We did not check the cell line identification by short tandem repeat profiling. The DH-14-3 cells have two karyotypes, but this cell line retained the properties of the thyroid gland.
To the best of our knowledge, only a few PDTC cell lines have been established. One PDTC cell line, SMP, secreted IL-1, IL-6, IL-8, and granulocyte colony-stimulating factor (Asakawa and Kobayashi 1999). Another PDTC cell line, NPA, had BRAF and p53 mutations and expressed EGFR and CXCR4 (Rodrigues et al. 2007). DH-14-3 cells express the chemokine receptor and produce hemangiogenic and mitogenic factors. Thus, DH-14-3 cell line may be useful for studies of PDTC.
PDTC preferentially manifests bone metastases. A highly metastatic breast cancer cell line (MDA-Met) and a well-characterized prostate tumor cell line (PC-3) with high bone metastatic potential were found to have high mRNA expression levels of IL-8 (Bendre et al. 2005; Lu et al. 2007). The IL-8 expression level correlated with the reported metastatic phenotype of the cell lines, which had an aggressive bone metastatic phenotype. These cell lines were generated by the repeated passage of less metastatic parental cell lines in vivo. The high constitutive level of IL-8 production in the newly established DH-14-3 cell line is of particular interest to us, since the patient, who had the origin tumor, had suffered from multiple bone metastases.
In this study, we demonstrate that DH-14-3 cells express CXCR4 and produce high levels of IL-8, MMPs, TGF-β1, VEGF, and PTHrP. These results raise the possibility that CXCR4-positive DH-14-3 cells preferentially metastasize to the bone marrow, and cytokines produced by the DH-14-3 cells sustain osteoclastic bone resorption and promote osteolytic bone metastasis. Thus, the DH-14-3 cell line provides an excellent tool to investigate the molecular and biological features of PDTC as well as the mechanisms of bone metastasis of thyroid carcinomas. This cell line can also provide a strategy to enhance therapeutic modalities of malignant thyroid diseases.
The authors would like to thank Mika Watanabe, F. Fujishima, and N. Ogawa for helpful discussion, and Y. Furukawa and Y. Takahashi for technical assistance.
We have no conflict of interest.