2013 年 230 巻 4 号 p. 191-196
2013 年 230 巻 4 号 p. 191-196
Hepatocellular carcinoma (HCC) is the third leading cause of cancer deaths worldwide. Serum albumin (Alb) is an important prognostic factor for patients with HCC. Moreover, plasma levels of branched-chain amino acids (BCAA), L-valine, L-leucine, and L-isoleucine, are commonly decreased in patients with cirrhosis. Accordingly, formulations of BCAA has been used to maintain the Alb level and prevent ascites in patients with cirrhosis. The aim of this study is to investigate differences in the changes in Alb between a group that received a BCAA formulation (n = 29) and a group given a standard diet (n = 60) in the course of HCC recurrences. All patients experienced more than one hospitalization (mean: 2.6; range: 2-10) owing to recurrence. The plasma BCAA concentration and BCAA-to-tyrosine ratio (BTR), which is a good indicator of the severity of hepatic parenchymal injury in patients with cirrhosis, were significantly correlated with Alb. We defined the changes in BCAA and Alb between recurrences as ΔBCAA and ΔAlb, respectively, and stratified the patients in both groups based on number of recurrences (3 < early, 3-5 middle, or 5 > later). There was also a positive correlation between ΔBCAA and ΔAlb. Interestingly, in the group with BCAA, ΔAlb and ΔBCAA were significantly smaller, especially in the middle period (3-5 recurrences), than in the group without BCAA. These results indicate that the BCAA supplementation could maintain the BCAA and Alb levels in the middle period (3-5 recurrences). BCAA formulation is useful for hypoalbuminemia in the course of HCC recurrence.
Hepatocellular carcinoma (HCC) is a primary malignancy of the liver. HCC is now the third leading cause of cancer deaths worldwide, with over 500,000 people affected (El-Serag and Rudolph 2007). The incidence of HCC is highest in Asia and Africa (Kiyosawa et al. 2004; Yuen et al. 2009). HCC is generally associated with liver cirrhosis. On the other hand, an imbalance of plasma amino acids, with decreased levels of branched-chain amino acids (BCAA), is commonly seen in patients with cirrhosis (Fischer et al. 1976), because the liver is the most important organ for maintaining nutritional homeostasis. The BCAA are among the nine essential amino acids for humans, accounting for 35% of the essential amino acids in muscle protein and 40% of the preformed amino acids required by mammals (Harper et al. 1984; Shimomura et al. 2004). BCAA have aliphatic side chains with a branch point, and comprise L-valine (Val), L-leucine (Leu), and L-isoleucine (Ile). In clinical situations, BCAA formulations are commonly used to maintain the Alb level (Nakaya et al. 2007) and prevent ascites in patients with cirrhosis, because BCAA promote Alb secretion through the mTOR signal pathway in liver (Matsumura et al. 2005). Long-term nutritional supplementation with oral BCAA has been shown to be useful to prevent progressive hepatic failure and to improve surrogate markers and the perceived health status (Marchesini et al. 2003). The European Society for Clinical Nutrition and Metabolism (ESPEN) recommends BCAA-enriched formulae for patients with hepatic encephalopathy (Plauth et al. 2006). Moreover, the oral administration of BCAA granules was reported to inhibit hepatic carcinogenesis in patients with compensated cirrhosis (Kobayashi et al. 2008). However, there is no report on whether a BCAA formulation maintains the BCAA and Alb levels in HCC patients with repeated therapy for recurrence. The aim of the study is to investigate the relation between HCC recurrence and changes in the BCAA and Alb levels in HCC patients undergoing repeated therapy.
We retrospectively enrolled 89 HCC patients who were admitted to Tohoku University Hospital from June 2005 to November 2012. Twenty-one patients were admitted for the first treatment for HCC, and then 68 patients were newly referred to this hospital for the treatment of recurrent HCC. All patients experienced more than one hospitalization (mean: 2.6; range: 2-10) owing to recurrence; thus, the total number of subjects was 233. We compared the changes in the plasma amino acids between a group that received a BCAA formulation (n = 29): hepatitis C virus (HCV) n = 26; hepatitis B virus HBV n = 1; and cryptogenic n = 2 and a control group that did not (n = 60): HCV n = 41; HBV n = 7; Alcoholic n = 8; nonalcoholic steatohepatitis NASH n = 1; Budd-Chiari syndrome n = 1; and cryptogenic n = 2, adjusted for age and Union for International Cancer Control (UICC) stage of HCC. In the BCAA group, 27 patients received 4.74 g of LIVACT (Ajinomoto Pharma, Tokyo, Japan) orally administered three times a day and 2 patients received orally administered Aminoleban EN (Otsuka Pharmaceutical Co, Tokyo, Japan) once a day. All patients received 30 kcal/kg/day meals (protein 1.1 g/kg and fat 0.7 g/kg) regardless of the administration of BCAAs. The patients received a medical examination including dynamic CT, dynamic MRI, abdominal ultrasonography, or a blood test at least every 3 months as outpatients at the hospital. The diagnosis of HCC was performed by the combination of dynamic CT (or dynamic MRI), and tumor markers (AFP and DCP). The modalities of treatment included transcatheter arterial chemoembolization (TACE), percutaneus ethanol injection (PEI), radiofrequency ablation (RFA), radiation therapy (RT), and hepatic resection (HR). If new lesions emerged during the follow-up periods, these subjects were hospitalized again and administered the same treatment.
AminogramThe concentrations of the plasma amino acids of fasting patients were measured by high-performance liquid chromatography (HPLC) in the early morning. Briefly, sulfosalicylic acid was added to plasma to a final concentration of 5%. The samples were then placed on ice for 15 minutes followed by centrifugation to remove precipitated proteins. The extracts were then analyzed for the amino acid content with a JLC-500/V (Japan Electron Optics Laboratories, Tokyo, Japan).
Statistical AnalysisComparison of the baselines of the two groups was made using the unpaired t-test, adjusted for age and UICC stage of HCC. All data are expressed as mean ± standard deviation (s.d.). The comparison of the biochemical data in each group was made by unpaired t-test. All statistical analyses were performed with standard statistical software (JMP® Pro 9 for Windows).
Eighty-nine patients were included in the present study, consisting of 29 patients in the BCAA group and 60 patients in the control group, adjusted for age and UICC stage of HCC (Table 1). The number of males, body weight, hemoglobin (Hb) level and Alb were significantly lower in the BCAA group, and the Child-Pugh score was significantly higher in the BCAA group. Accordingly, patients in the BCAA group had suffered from more progressive cirrhosis, compared with the control group. We confirmed that the concentration of BCAA was significantly and positively correlated with the concentration of Alb (r = 0.43 / P < 0.0001) (Fig. 1A). All three BCAA, L-Val, L-Leu and L-Ile, were positively correlated with the concentration of Alb (L-Val r = 0.44 / P < 0.0001, L-Leu r = 0.43 / P < 0.0001, L-Ile r = 0.42 / P < 0.0001). The BCAA-to-tyrosine ratio (BTR) is a good indicator of the severity of liver injury (Kawamura-Yasui et al. 1999). The BTR was also positively correlated with the concentration of Alb (r = 0.58 / P < 0.0001) (Fig. 1B).

Characteristics of enrolled patients with HCC.
Values are mean ± s.d.
BMI, body mass index (weight (kg) / height2 (m)); FBS, fasting blood sugar; C-P score, Child-Pugh score; BCAAs, branched-chain amino acids (L-Valine + L-Leucine + L-Isoleucine).
*p < 0.01.
Using unpaired t-test, adjusted for age and UICC stage.

The concentration of BCAA is significantly and positively correlated with the concentration of albumin.
The correlation was examined by regression analysis between Alb and BCAA (A), between Alb and BTR (B). Ellipsoids reflect the correlation envelopes for the cut-off (0.95) in the total population, and r represents coefficient of determination. The dots represent the values from BCAA-supplemented group (n = 80) and the circles represent the values from the control group (n = 153).
We defined the changes in BCAA and Alb between recurrences as ΔBCAA and ΔAlb, respectively, and stratified the changes on number of recurrences (3 < early, 3-5 middle, 5 > later) (Fig. 2A). Both ΔBCAA and ΔAlb were no significantly different among the recurrence times. In the early period, there was more treatment with RFA than during the other periods (Fig. 2B) and the recurrence interval was significantly longer (Fig. 2C). BCAA, Alb, ΔBCAA and ΔAlb were not different among the treatments for HCC (data not shown). Among the three periods, the concentration BCAA was significantly decreased in the later period (Fig. 2D). There was a positive correlation between ΔBCAA and ΔAlb (r = 0.16 / p = 0.045) (data not shown). Interestingly, in the group that received BCAA supplement ΔAlb and ΔBCAA were significantly smaller, especially in the middle period, than in the group without BCAA supplement (ΔAlb: mean change −0.02 vs. −0.20 mg/dL; p = 0.027 / ΔBCAA: mean change −10.7 vs. −42.8; p = 0.029) (Fig. 2E). Consistent with ΔBCAA, in the group that received BCAA supplement, the body weight change (ΔBW) tended to be smaller in the middle period than in the group without BCAA supplement (ΔBW: mean change 0.24 vs. −0.89; p = 0.14).
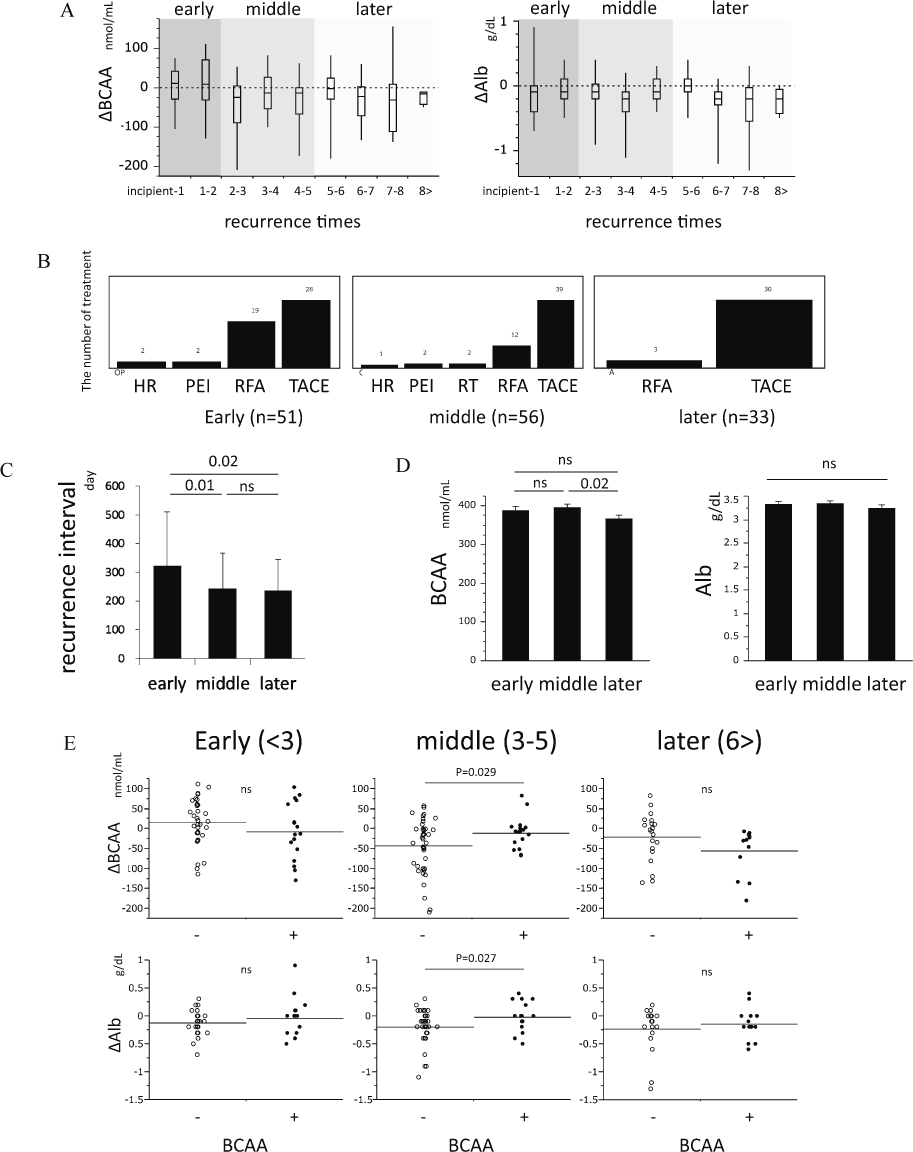
The BCAA supplementation prevents the decreases in serum albumin in repeated therapy of HCC.
(A) The changes of BCAA (left) and Alb (right) during HCC recurrences. We stratified the changes of BCAA and Alb based on number of recurrences (< 3 early, 3-5 middle, and 5 > later). The box plot provides median values (the line across the box), with the upper and lower quartile (the upper and lower border of the box), as well as the largest and lowest value in the set (the ends of the vertical line). Abscissa indicates recurrence times; for example, 1-2 indicates the period between the day of first recurrence and the day of second recurrence. The numbers of patients were 21 for incipient-1, 30 for 1-2, 26 for 2-3, 19 for 3-4, 11 for 4-5, 10 for 5-6, 11 for 6-7, 8 for 7-8, and 4 for 8 >. (B) Treatment differences among the three periods. (C) Recurrence interval among three periods: early n = 51, middle n = 56, and later n = 33. (D) The comparison of BCAA and Alb among the three periods: early n = 60, middle n = 108, and later n = 65. (E) The changes of BCAA and Alb were compared between the two groups. The horizons represent each average. The thick lines or dots represent the changes from BCAA-supplemented group (early n = 17, middle n = 16, and later n = 13) and the thin lines or circles represent the changes from the control group (early n = 34, middle n = 40, and later n = 20).
Recently, some prospective clinical studies demonstrated that BCAA formulations can prevent HCC carcinogenesis (Kobayashi et al. 2008) and recurrence (Ichikawa et al. 2012a). The mechanism that underlies these phenomena is thought to involve immunity (Kakazu et al. 2007; Kakazu et al. 2009; Honda et al. 2011), antioxidant (Ichikawa et al. 2012b), antiapoptotic (Kuwahata et al. 2012) and other factors (Yoshiji et al. 2009; Hagiwara et al. 2012; Miuma et al. 2012; Shimizu et al. 2012). However the mechanism still remains largely unknown. In this study, the recurrence interval was not different between the BCAA group and control group (data not shown), but the interval was significantly longer in the early period than in the other periods (Fig. 2C). The reason for this is that, RFA, which has high curability, was used more often in the early period than during other periods. On the other hand, it is well known that BCAA formulations are effective for maintaining the Alb level in patients with cirrhosis (Nakaya et al. 2007). However, there is no report in which multiple measurements of BCAA and Alb were performed in matched HCC patients with recurrence. In this study, we revealed that BCAA formulations could prevent a decrease in the BCAA and Alb, especially in the middle period (3-5 recurrence times). In the other periods, these changes were not significantly different between the two groups. Among the three periods, the C-P score was not different (early: 6.0 ± 0.94; middle: 6.1 ± 1.06; latter: 6.1 ± 1.05), nevertheless the UICC stage was significantly different (early: 1.4 ± 0.65; middle: 2.0 ± 0.89; latter: 2.4 ± 0.88). Furthermore, the concentration of BCAA was significantly decreased in the later period (Fig. 2D). One should consider the possibility that, in the early period, the BCAA and Alb levels were maintained regardless of BCAA supplementation because the development of HCC was weak. However, in the later period, the Alb and BCAA decreased regardless of BCAA supplementation because the development of HCC was strong. There were some limitations in the present study, including the small number of subjects and lack of a randomized controlled design. Furthermore, the liver function, especially Alb, was more impaired in the BCAA group than control group, because BCAA formulation is commonly used for the patient with advanced cirrhosis. We think that the liver function of patients in the BCAA group will be worse without BCAA supplementation. To demonstrate this possibility, we need to perform a large scale, randomized, controlled trial in a well-characterized group of patients with appropriate nutritional evaluation and determine the effects of maintaining the Alb level in a longitudinal follow-up. Furthermore, carcinogenesis from nonalcoholic steatohepatitis (NASH) has been increasing, recently (Ascha et al. 2010). The weight, plasma BCAA and serum Alb in NASH patients were significantly higher than those in patients with other types of chronic hepatitis (Kakazu et al. 2013). To account for this paradox, we need to measure the concentration of BCAAs after classification by disease progression in NASH because, in this study, the major etiology of HCC was chronic hepatitis C. The Child-Pugh grade is very important for deciding the treatment strategy for HCC (Kudo et al. 2011), and the Alb level is the most variable factor in the classification. Therefore, preventing decreases in Alb is very important for improving the prognosis of patients with HCC, because therapeutic options are extensive. In summary, we have shown that the BCAA concentration was significantly and positively correlated with the Alb in patients with HCC, and the BCAA formulations were useful for maintaining the Alb levels in repeated therapy for HCC, especially in the middle period (3-5 recurrences). Our data concerning BCAA and Alb provide the rationale for future nutrition therapy, which could be beneficial to patients with HCC.
This work was supported by Grant-in-Aid for Young Scientists (B) No. 23790762 from the Ministry of Education, Culture, Sports, Science and Technology of Japan, and supported in part by MEXT Tohoku Medical Megabank Project.
The authors have no financial conflict of interest.