2013 Volume 231 Issue 1 Pages 9-12
2013 Volume 231 Issue 1 Pages 9-12
Mitochondrial disorder (MtD) is usually a multisystem disease due to impaired mitochondrial energy production. Severe hypokalemia resulting in muscle weakness and rhabdomyolysis has not been reported as a phenotypic feature of MtD. Here we describe a 60-year-old male patient who developed myalgias followed by generalized muscle weakness a few days before admission. Symptoms were attributed to severe hypokalemia that occurred after the patient had discontinued spironolactone, a competitive antagonist of the aldosterone receptor, four months earlier on his own judgment. Spironolactone was given for 10 years to treat suspected primary hyperaldosteronism (Conn’s syndrome). He presented with myopathic face, bilateral ptosis, hypertelorism, brachydactylia, weakness of the axial and limb muscles, and bilateral leg edema. Hypertelorism and brachydactylia are known as physical traits of MtD. Laboratory investigations revealed hypokalemia of 1.7 mmol/l and elevated serum levels of creatine kinase (2,772 U/l). Electrocardiogram showed sinus rhythm, left bundle-branch-block, repolarization abnormalities, and prolonged QTc (571 ms), which is associated with a propensity to ventricular arrhythmias. Diagnostic work-up revealed bilateral adenomas of the suprarenal glands. Conn’s syndrome was regarded as a manifestation of MtD, since MtDs are frequently associated with endocrine abnormalities. The patient also presented with occasional double vision, ptosis, renal insufficiency, bilateral renal cysts, hypertriglyceridemia, arterial hypertension, and hypertrophic cardiomyopathy. Taken together, we have made the diagnosis of MtD. In conclusion, MtD may be associated with adrenal adenomas, which may cause severe symptomatic hypokalemia, manifesting as generalized weakness and myalgias due to rhabdomyolysis. Endocrine involvement may be a phenotypic feature of MtD.
Mitochondrial disorder (MtD) is most frequently due to impaired energy production by the respiratory chain or the oxidative phosphorylation. The biochemical defect may result from mutations in the mitochondrial DNA or the nuclear DNA. MtD may manifest as a syndromic or non-syndromic disorder, or as a mono-organ or multi-organ disease (Finsterer 2012). Hypertelorism and brachydactylia were reported as physical traits of MtD (Pronicki et al. 2002; Finsterer 2011). Furthermore, hypokalemia has been occasionally described as a manifestation of a syndromic, multisystem MtD (Wang et al. 2000; Menegon et al. 2004; Emma et al. 2006), particularly in Kearns-Sayre syndrome (KSS) (Goto et al. 1990; Harvey and Barnett 1992; Park et al. 2004; Emma et al. 2006). However, severe hypokalemia resulting in muscle weakness and rhabdomyolysis has not been reported as a phenotypic feature of a non-syndromic MtD.
The patient is a 60-year-old Caucasian male, height 179 cm, weight 100 kg, who developed myalgias starting on the thighs, followed by generalized, progressive muscle weakness a few days before admission due to severe hypokalemia after having discontinued spironolactone, a competitive antagonist of the aldosterone receptor, and reduced potassium substitution four months earlier. Spironolactone was given for 10 years to treat suspected primary hyperaldosteronism (Conn’s syndrome), because of elevated aldosterone (217 pg/ml). However, the patient had stopped spironolactone on his own judgement, since his potassium values were normal and since he believed that he required a drug holiday. The presence of generalized weakness and myalgias suggested rhabdomyolysis.
Ten years ago (in 2003), the patient had presented with transient double vision and ptosis, elevated serum levels of creatine kinase (CK), arterial hypertension, transient renal insufficiency, and mild, intermittent hypokalemia (Table 1). Cerebral MRI only showed T2-weighted hyperintense lesions within the white matter periventricularly, and small hypointense lesions within the basal ganglia. The corpus callosum was normal in shape and size. Echocardiography revealed marked concentric hypertrophy (septum: 19 mm), a restrictive filling pattern, and a slightly enlarged left atrium. MRI of the suprarenal glands performed in 2003 revealed only a single renal cyst on the right side. No suprarenal gland adenoma was found at that time. Hypokalemia was recognized for the first time already 15 years ago (1998). Arterial hypertension was diagnosed 15 years ago (1998) and psoriasis 10 years ago (2003). He did not report dysphagia, dysarthria, or tremor. The family history was negative for primary hyperaldosteronism. He has three healthy children.
Clinical neurologic examination on admission in 2013 revealed myopathic face with bilateral ptosis, hypertelorism, brachydactylia, weakness of the axial and limb muscles, and bilateral leg edema. There was no ophthalmoparesis or alternating exotropia. The lower cranial nerves were intact. Tendon reflexes were preserved and there was no tremor or other cerebellar deficit, or dyskinesias. Laboratory investigations revealed hypokalemia of 1.7 mmol/l (normal values, 3.3-5.1 mmol/l) and CK elevation to 1,522 U/l (> 175 U/l) (Table 1). Other electrolytes and renal function parameters were intact (Table 1). Potassium in the urine, obtained on the 2nd day after admission, was reduced to 9.9 mmol/l (20-80 mmol/l). Electrocardiogram (ECG) on admission showed sinus rhythm, a PQ interval of 0.2 ms, a QRS duration of 0.12 ms, left bundle-branch-block, and depolarization abnormalities (ascending ST elevation in V1-3, descending ST depression in V4-6), and a prolonged QTc (571 ms). MRI of the suprarenal glands in 2013 revealed an adenoma of 15 mm in diameter on the left side, an adenoma on the right side, and multiple renal cysts bilaterally. Angiography of the inferior caval vein to take multiple blood samples from the renal and suprarenal veins revealed particularly elevated serum aldosterone levels in venous blood from the left renal and suprarenal vein, but also from the caval vein (Table 2). The diagnosis of Conn’s syndrome was confirmed based upon elevated serum aldosterone levels, low serum renin levels, and the suspected suprarenal adenomas on MRI. Needle electromyography (needle-EMG) to record the electrical activity of the right vastus lateralis and the left anterior tibial muscles was normal.
Hypokalemia was treated by continuous, rapid substitution of potassium intravenously and restarting spironolactone. Under this regimen the described symptoms and signs gradually recovered to the pre-morbid status and serum potassium values continuously increased to become normal within 18 h (Table 1). ECG recorded on the 2nd day after admission showed sinus rhythm, a PQ interval of 0.2 s, a QRS duration of 0.1 s, a normal ST segment, normal T wave, and a QTc of 429 ms. Serum CK increased to a maximal value of 2,772 U/l and slowly normalized thereafter again. Myopathic face and ptosis remained unchanged after recovery. He was scheduled for surgical removal of the adenomas.

Most important blood chemical values during the last ten years.
GFR, glomerular filtration rate; BUN, blood urea nitrogen; CK, creatine kinase; CK-MB, myocardial type of CK; nd, not done. The data of 2013 are given in bold.
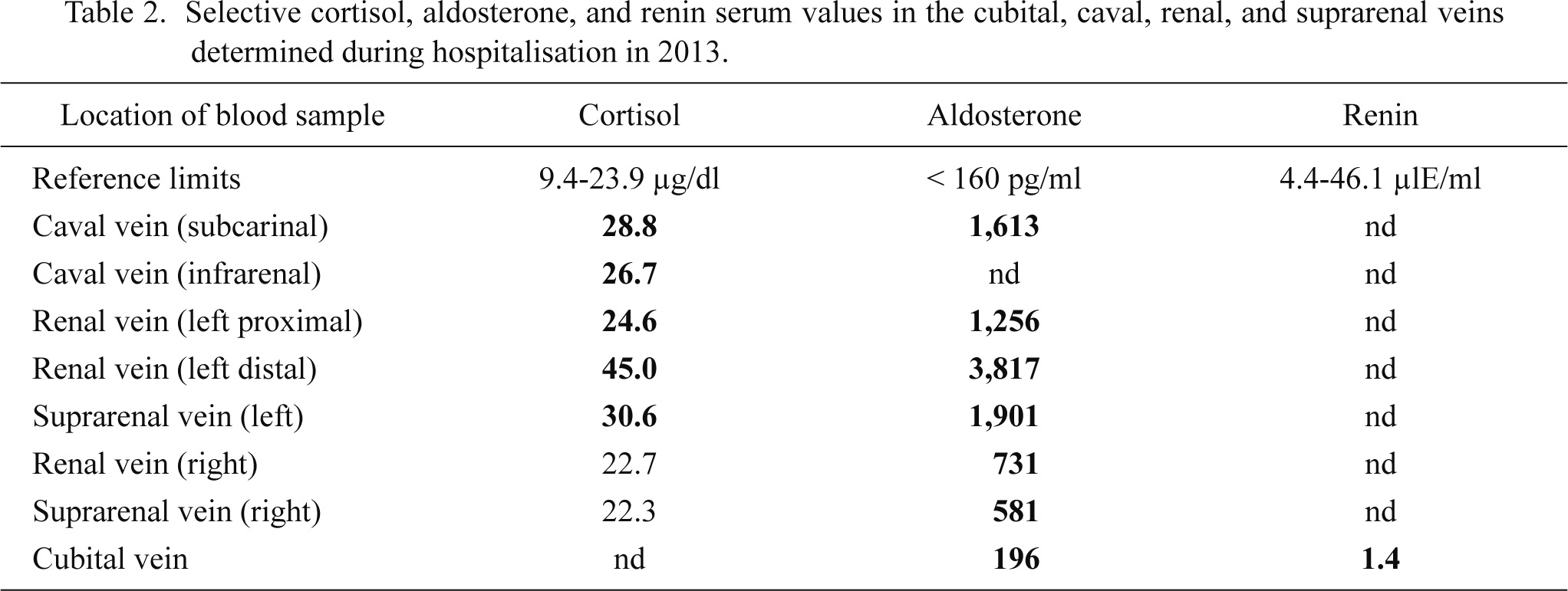
Selective cortisol, aldosterone, and renin serum values in the cubital, caval, renal, and suprarenal veins determined during hospitalisation in 2013.
Abnormal values are given in bold.
The presented patient showed severe hypokalemia of 1.7 mmol/l associated with generalized muscle weakness, rhabdomyolysis, and ECG abnormalities. Severe hypokalemia was attributed to Conn’s syndrome and discontinuation of spironolactone given for bilateral suprarenal gland adenoma. The suprarenal adenomas and Conn’s syndrome were regarded as manifestations of a suspected MtD. MtD was suspected because of the clinical presentation with occasional double vision and ptosis since 10 years earlier, hypertelorism, brachydactylia, renal insufficiency, bilateral progressive renal cysts, the suprarenal adenomas, hypertriglyceridemia, arterial hypertension, hypertrophic cardiomyopathy with a restrictive filling pattern, and psoriasis. Left ventricular hypertrophy was attributed to the suspected genetic defect because blood pressure was well controlled, hypertrophic cardiomyopathy has been reported as a manifestation of MtD (Palecek et al. 2012; Vondráčková et al. 2013), and because left ventricular hypertrophy was extensive. Hypertelorism and brachydactylia were also suggestive of MtD since they have been reported as MtD manifestations (Pronicki et al. 2002; Finsterer 2011). The diagnosis could not be confirmed by muscle biopsy, biochemical investigations of the muscle, or genetic tests, since the MtD was suspected only retrospectively and the patient repeatedly did not consent with further investigations. MtD was suspected despite the negative family history for MtD, based upon the multisystem nature of the patient’s disease, and a constellation of abnormalities compatible with MtD. Hypertrophic cardiomyopathy, renal insufficiency, endocrine abnormalities, and dermal problems are typical clinical features suggestive of MtD.
Further arguments for a non-syndromic MtD are that hypokalemia or hyperaldosteronism has been previously reported as clinical manifestations of MtD (Goto et al. 1990; Harvey and Barnett 1992; Wang et al. 2000; Menegon et al. 2004; Park et al. 2004; Emma et al. 2006). Emma et al. (2006) reported a 14-year-old patient with KSS who developed Bartter’s syndrome (hyper-reninemic hyperaldosteronism). Renal involvement was attributed to predominantly high heteroplasmy in aldosterone-responsive tubular structures resulting in the development of hypokalemic alkalosis (Emma et al. 2006). Recurrent paralysis was also reported in a patient with non-syndromic MtD carrying multiple mtDNA deletions (Prelle et al. 1993). Hyperaldosteronism was reported as one among various other phenotypic features in a single male with KSS (Mihai et al. 2009). Menegon et al. (2004) described a patient with atypical Bartter’s syndrome and MtD who presented with salt and volume depletion, hypotonia, and secondary hyperaldosteronism associated with hypokalemic metabolic alkalosis, hypocalcaemia and severe hypomagnaesemia. These abnormalities were attributed to mitochondrial dysfunction within the epithelium of the ascending loop of Henle (Menegon et al. 2004). In a 9-year-old male patient, hypokalemia and hypophosphatemia triggered severe proximal muscle weakness from mitochondrial myopathy (Wang et al. 2000). In a 10-year-old male with KSS due to a large-scale 8.8-kb mtDNA deletion, renal involvement manifested as tubular dysfunction with excessive excretion of potassium and magnesium associated with hyper-reninemia and hyperaldosteronism (Bartter’s syndrome) (Goto et al. 1990). Renal dysfunction was attributed to a defect of the thick ascending limb of the loop of Henle (Goto et al. 1990). Hyperaldosteronism was also reported in other KSS cases (Harvey and Barnett 1992; Park et al. 2004). Electrolyte disturbances in MtDs are particularly frequent if there is renal or suprarenal involvement (Sekine 2002). Most frequently, however, hyperaldosteronism has been reported in association with KSS.
The present patient shows that MtD may manifest with adenomas of the suprarenal glands and that these adenomas may cause severe symptomatic hypokalemia, manifesting as generalized weakness and myalgias due to rhabdomyolysis. Endocrine involvement may be a rare phenotypic feature of MtD.
The authors declare no conflict of interest.