2013 Volume 231 Issue 2 Pages 101-110
2013 Volume 231 Issue 2 Pages 101-110
Autologous fat transplantation (AFT) is a common and important operation in plastic surgery for soft tissue defects and adipose tissue-derived stem cells (ADSCs) are considered as a promising supplement to decrease absorption and subsequent side effects due to the ability of multiple differentiation and production of vascular endothelial growth factor (VEGF). The capacities of ADSCs can be further enhanced by treatment with 17-β estradiol (E2). Therefore, we hypothesized that E2 may promote the potential of ADSCs for AFT. In this study, ADSCs were extracted from three female patients by liposuction. In vitro studies showed that E2 supplementation at an optimal concentration of 10-8 M resulted in enhanced proliferation, VEGF production, and adipogenic differentiation of human ADSCs, and reduced apoptosis rate in a serum-free environment. In addition, a nude mice model of fat transplantation was utilized to demonstrate the efficacy of ADSC for survival ratio in vivo. These results using the volume of fat tissues after 12 weeks compared original volume, revealed that the addition of E2-treated ADSCs induced a significantly higher tissue survival ratio (76.9 ± 1.9%) when compared with the ADSC-free system (55.5 ± 1.5%). Furthermore, increased capillary formation stained with hematoxylin-eosin (H&E) was observed in ADSCs systems after treatment with E2. Therefore, this study demonstrated E2 could promote the capacities of ADSCs about aspects of adipogenic differentiation, growth factor secretion and apoptosis reduction in vitro, vascularization improvement in vivo, and then enhanced the survival ratio of AFT.
Autologous fat transplantation (AFT) shows great potential in repairing soft tissue defects caused by trauma, tumor resection and congenital anomalies due to incisional scar-free and minimal complications (Spear et al. 2005; Coleman and Saboeiro 2007). Furthermore, AFT has been considered as a promising alternative to artificial tissue implants (Dragoo et al. 2003; Knippenberg et al. 2005). However, a low survival ratio and subsequent side effects, such as necrosis and calcification, result in the unpredictable degree of absorption which limits its clinical efficacy and further applications (Chajchir et al. 1990; Lu et al. 2009).
A significant aspect of adipose tissue engineering is to reduce the fat absorption and to retain its predesigned physical contour and dimension after transplantation (Karacaoglu et al. 2005; Yamaguchi et al. 2005; Coleman and Saboeiro 2007; Missana et al. 2007; Ogawa et al. 2007). Yoshimura and co-workers recently demonstrated that adipose derived stem or stromal cells (ADSCs)-enriched AFT was efficient in breast augmentation and facial lipoatrophy, with stable natural contour and without any major side effects (Yoshimura et al. 2008a, b). ADSCs can differentiate into different types of cells such as adipocytes, osteoblasts, endothelial cells, and chondrocytes (Zuk et al. 2001; Cowan et al. 2004; Hong et al. 2007). ADSCs are considered to reside between adipocytes (differentiated from ADSCs) or in the extracellular matrix (ECM), as well as to induce adipose tissue formation within 6 months after implantation (Strawford et al. 2004; Yoshimura et al. 2008a). Moreover, the ability of growth-factor release of ADSCs promotes adipose tissue formation (Rehman et al. 2004). Given that normal turnover of adipose tissue takes at least 2 years (Strawford et al. 2004), the use of ADSCs has great superiority and potential in adipose tissue engineering.
Compared with bone marrow mesenchymal stem cells (MSCs), which have been extensively studied for stem cell-based therapies, the use of ADSCs in tissue repair and regeneration is further encouraged by its abundant existence in the human body and its easy-availability with negligible donor-site morbidity and patient discomfort (Xu et al. 2007; Lu et al. 2009). However, ADSCs exhibit lower proliferation and differentiation activities compared to MSCs (De Ugarte et al. 2003; Huang et al. 2005), requiring further improvement in this area.
Sexual steroid estrogens play a significant role in modulating the growth, differentiation and metabolism of many tissues and cells (Cooke and Naaz 2004; Dang and Lowik 2004; Heim et al. 2004; Talwar et al. 2006). Among them, 17-β estradiol (E2) has been proved to be effective in promoting osteogenesis and adipogenesis of human bone marrow MSCs and to enhance vascular endothelial growth factor (VEGF) production due to the binding between E2 and estrogen receptors (ERs) (Hong et al. 2006). A previous study indicated the presence of ERs in adipose tissues (Hong et al. 2007). Therefore, in this study, we investigated the effects of E2 on proliferation of ADSCs and on VEGF secretion in vitro. In addition, the advantage of E2-treated ADSCs in AFT was demonstrated.
Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, Utah, USA) supplemented with 10% fetal bovine serum (FBS, Hyclone, USA) and 1% antibiotic-antimycotic (Sigma, New Jersey, USA) was prepared as standard culture medium. Adipogenic differentiation medium was prepared as DMEM supplemented with 10% FBS, 1 μM dexamethasone (Sigma, New Jersey, USA), 10 μg/mL insulin (Sigma, New Jersey, USA), 200 μM indometacin (Sigma, New Jersey, USA) and 0.5 mM isobutyl-methylxanthine (IBMX, Sigma, New Jersey, USA). Phosphate buffered saline (PBS) was purchased from HaiGene Corp (Shanghai, China). Human umbilical vein endothelial cells (HUVECs) were supplied by the 4th Affiliated Hospital of Harbin Medical University. All the other reagents were purchased from Sigma except when mentioned.
Patients and specimensBoth experimental and clinical protocols were approved by the Medical Ethical Committee 1st Affiliated Hospital of Harbin Medical University. Written consents were taken from all the patients involved in this study.
Adipose tissue was harvested from four female outpatients (aged from 22 to 30 years old) who underwent thigh liposuction in the Plastic and Cosmetic Center from September 2011 to March 2012.
AnimalsTwenty-one female nude mice (5 weeks old, 18-20 g) were obtained from Slac Laboratory Animal (Shanghai, China). All the animals were cultivated and operated under the permission of Harbin Veterinary Research Institution of Chinese Academy of Agricultural Sciences.
Isolation of human ADSCsAdipose tissues were washed three times with PBS to remove blood cells and then digested with an equivalent volume of 0.1% collagenase type I while vigorously shaking at 37°C for 45 min. After centrifugation at 1,000 rpm for 10 min, the pellet was re-suspended in DMEM and filtrated through a mesh with a pore size of 100 μm. After filtration, a second centrifugation at 1,000 rpm for 10 min was performed, and the harvested cells (pellet) were re-suspended in culture medium in a Petri dish at a density of 8,000 cells /cm2 and maintained in humidified air with 5% CO2 at 37°C. The culture medium was replaced every 3 days. The cells were trypsinized, counted, and subcultured at a ratio of 1:3 upon reaching 80-90% confluence. Cells of passage three (P3) were used in further studies.
The effect of E2 on proliferation of human ADSCsThe cells were trypsinized, collected at a concentration of 3 × 104 cells/mL, and randomly divided into 6 groups in a 96-well plate. The cells were incubated in culture medium, allowing for adhesion (6 h). Each group was then supplemented with different concentrations of E2 from 10−6 M to 10−10 M and incubated for 24 h. Finally the medium was replaced by fresh culture medium for further culture for 2 and 4 days, respectively. A Cell Counting kit-8 (cck-8, Dojindo, Japan) was used to evaluate the cell proliferation rate according to the manufacturer’s instructions. As a control, human ADSC proliferation without E2 was carried out in the same manner.
The effect of E2 on VEGF production in human ADSCsADSCs were plated in 24-well plates at a concentration of 1 × 105 cells/mL for 6 h. Then, E2 was supplemented with different concentrations from 10−6 M to 10−10 M. After 24 h, supernatant of each plate was harvested and centrifuged for a subsequent enzyme-linked immunosorbent assay (ELISA, R&D Systems, Minnesota, USA) to measure the VEGF concentration.
HUVEC is a kind of vascular endothelial cell (VEC), and amount of VEGF is proportional to HUVEC proliferation. A HUVEC model in vitro also imitates proliferation of host VEC in the environment of VEGF secretion from different group (treated ADSCs, untreated ADSCs and culture medium only) in nude mice, as detailed below. It may reflect the results of treated and untreated ADSCs in tissue vascularization in vivo. In brief, HUVECs (passage 2, 5 × 104 cells/mL) were cultured in special culture medium (VEGF-containing supernatants of ADSCs harvested in the test above), and stimulated with E2 at different concentrations (10−10 M to 10−6 M) in a 96-well plate for 48 h, then a Cell Counting kit-8 was used again to evaluate the HUVEC proliferation. The numbers of HUVEC of each group were demonstrated by the optical density (O.D.) value at 490 nm.
Adipogenic differentiation of E2-treated human ADSCsHuman ADSCs were cultured in basic medium, and upon 70% confluence, DMEM was replaced by the adipogenic differentiation medium for an additional 14 days. According to the results of E2 on proliferation and VEGF production of ADSCs, E2 supplementation at concentrations of 10−10 M, 10−8 M and 10−6 M was chosen to demonstrate the effect of E2 on the adipogenic differentiation of human ADSCs.
Oil-O-Red stainingAfter a 2-week culture in adipogenic differentiation medium, human ADSCs were rinsed twice with PBS, fixed in 10% neutral buffered formalin for 30 minutes, and then immersed in 1.5 mL 60% Oil-O-Red solution for 20 min. Stained human ADSCs were visualized under a light microscope (Olympus) equipped with a digital camera (Sony). The images were analyzed using Image Pro Plus 6.0 software. To estimate the efficacy of adipogenic differentiation of human ADSCs modulated by different concentrations of E2, the stained and unstained cells were counted in accordance with ten images randomly taken at each concentration. The positive ratio was calculated as the number of stained cells/number of total cells.
Reverse transcriptase polymerase chain reaction (RT-PCR) analysisTo investigate the adipogenic differentiation potential of human ADSCs, total RNA was extracted using TRIzol reagent (HaiGene Corp., Shanghai, China) from cells cultured in normal culture medium, adipogenic differentiation medium and adipogenic differentiation medium with E2 (10−8 M). The extracted RNA was converted to single-stranded cDNA using a commercial cDNA synthesis kit (HaiGene Corp., Shanghai, China). Ampli-Taq DNA polymerase (Applied Biosystems, California, USA) was used to amplify aliquots of the cDNA for peroxisome proliferator-activated receptors-γ2 (PPAR-γ2) and lipoprotein lipase (LPL). The housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a control for RNA loading samples. The reactions were carried out as follows: 95°C for 2 min; 95°C for 10 s; 60°C for 10 s; 72°C for 30 s; and followed by one cycle of 72°C for 2 min. PCR products were analyzed electrophoretically with an Agilent 2,100 bioanalyzer (Agilent Technologies, Palo Alto, California, USA). All specific primer sequences are summarized in Table 1.

PCR Primers for adipogenic differentiation markers.
Human ADSCs (P3) were divided into three groups (control, serum-free and serum-free + E2) for further incubation. In the control (standard culture medium) and serum-free groups (standard culture medium without serum), the cells were incubated in culture medium for 24 h. In the serum-free + E2 group (standard culture medium without serum but supplement with E2), the culture medium was supplemented with 10−8 M E2. After a PBS rinse, cells in the control group were kept in standard culture medium (with FBS), while cells in the serum-free and serum-free + E2 groups were incubated in DMEM (without FBS) for 48 h in humidified air with 5% CO2 at 37°C.
The cells were then trypsinized and collected at a concentration of 1 × 106 cells/mL for the Annexin V/propidium iodide (PI) apoptosis detection kit (BD Pharmingen, San Diego, USA) according to manufacturer’s instructions. The treated cells were analyzed by a flow cytometer.
In vivo fat transplantationHuman ADSCs were cultured in DMEM supplemented with E2 (10−8 M). After 24 h, the supernatant was removed, and the cells were washed with PBS to eliminate any residual E2. The cultured human ADSCs were then trypsinized and re-suspended in culture medium with a concentration of 5 × 106 cells/mL. Adipose tissue (0.3 mL) isolated from one of the patients was mixed with 0.2 mL of E2-treated ADSCs (group a), an untreated ADSCs mixture (fresh cultured human ADSCs, group b), and culture medium only (group c), respectively. The mixture was injected subcutaneously at three spots on the back of each nude mouse as shown in Fig. 5A. After 12 weeks, 21 nude mice were sacrificed and a total of 63 fat transplants were collected for further analysis.
The survival ratio of the harvested tissues was calculated by using method as previously published (Lu et al. 2009). In brief, the harvested tissue at each condition was suspended in 0.2 mL PBS in graduated containers. After stabilization, the mixture was extracted by using a needle syringe to achieve that the final volume remained in container was 0.2 mL. The survival rate was calculated as the ratio of extracted tissue volume over the original adipose tissue volume (the original volume is 0.3 mL as introduced in the previous paragraph).
Finally, the collected tissues were fixed in 10% neutral buffered formalin and embedded in paraffin. Tissue sections from the center of tissue biopsy were then stained with hematoxylin-eosin (H&E) and examined under a light microscope.
Statistical analysisEach test was repeated six times except when mentioned. Statistical significance was determined by an independent Student’s t-test using SPSS 17.0 software. Data are represented as mean ± SEM. A confidence level of 95% was considered statistically significant.
The isolated (undifferentiated) human ADSCs with a yield of 1 × 106 cells per 20 mL adipose tissue exhibited a fibroblast-like morphology (Fig. 1A, left), and this morphology could be maintained up to passage 20.
Oil-O-Red staining was used to further demonstrate the effect of E2 treatment on the differentiation ability of human ADSCs (Fig. 1A middle and right). Untreated human ADSCs exhibited a positively staining ratio of 75.9 ± 0.6%. Upon E2 supplementation, the ratio of Oil-O-Red positive cells increased to 77.0 ± 1.2% (10−6 M), 79.6 ± 0.8% (10−8 M) and 77.5 ± 0.8% (10−10 M), respectively, as shown in Fig. 1B. However, only E2 at a concentration of 10−8 M showed statistical significance (p < 0.001).

Images of ADSCs.
A: Microscope images of undifferentiated ADSCs at passage 3 (left panel) and Oil-O-Red stained adipocytes differentiated from human ADSCs treated with medium without E2 (middle panel) and with E2 at a concentrations of 10−8 M (right panel). B: The positive stained ratio following treatments with different concentrations of E2. Student’s t-tests were used to compare the positively stained ratio of ADSCs treated with different concentrations of E2 with that of the control group (undifferentiated ADSCs) to indicate statistical significance. ***p < 0.001.
Fig. 2A illustrates the dose-dependent effect of E2 supplementation on the proliferation of human ADSCs on day 2 and day 4. On day 2 post-culture, ADSCs after treatment of E2 (with a concentration of 10−8 M) promoted cell proliferation. No statistical difference was found between other E2 concentrations compared to control. Upon prolonged culture (4 days), the effect of E2 (10−8 M) was more pronounced (p < 0.01). E2 at a concentration of 10−10 M also showed significant beneficial effects in cell proliferation (p < 0.01) on day 4 post-culture.

E2 treatment on in vitro ADSC and HUVEC proliferation and VEGF expression.
A: Human ADSC proliferation (optical density (O.D.) at 490 nm) after E2 treatment at different concentrations at day 2 and day 4, respectively. B: VEGF expression of human ADSCs after treatment with E2 at different concentrations. C: HUVEC proliferation (O.D.) upon incubation in culture medium with different concentrations of E2. D: the correlation between VEGF concentration and HUVEC proliferation (O.D.). Student’s t-tests were used to compare the O.D. (490 nm) value of ADSCs and HUVECs, as well as the VEGF concentration and of ADSCs treated with different concentrations of E2, with those of the control group (E2-free) to indicate statistical significance. *p < 0.05; **p < 0.01.
The results of the ELISA demonstrated that human ADSCs incubated in the presence of 10−8 M E2 showed significantly higher VEGF production (1,503 ± 27 pg/mL) than control group (1,101 ± 55 pg/mL). E2 at other concentrations demonstrated no significant differences when compared to control (Fig. 2B).
Amount of VEGF is proportional to HUVEC proliferation, and therefore, VEGF-containing medium was used for HUVEC culture. As shown in Fig. 2C, E2-treated human ADSCs at a concentration of 10−8 M exhibited a significantly higher HUVEC proliferation ability when compared to the E2-free group (control). In contrast, at other E2 concentrations, negligible differences in HUVEC proliferation were found. These results are in agreement with the experiments investigating VEGF production. It reminds us HUVEC proliferation may be promoted by more amount of VEGF in the group ADSCs treated with E2 at a concentration of 10−8 M rather than in the group (untreated ADSCs) in the vivo test.
Adipogenic differentiation of E2-treated human ADSCsHuman ADSCs were able to differentiate towards adipocytes in adipogenic medium. The presence of E2 could further enhance the differentiation efficiency. After a 4-day culture, human ADSCs were circular shape, rather than their normal long spindle-like shape. In addition, there were some lipid droplets with brightness in the cytoplasm. After 2 weeks, bead-like lipid droplets were found in most cells, and the volume appeared to be enlarged to almost all the cells.
The expression of LPL and PPAR-γ2, characteristic genes of adipocytes, were evaluated using RT-PCR followed by quantitatively intensity analysis. As shown in Fig. 3, the expression of LPL and PPAR-γ2 was up-regulated in ADSCs when exposed to adipogenic differentiation medium, while nearly no expression was found following exposure to culture medium. E2 treatment medium resulted in higher gene expressions of LPL and PPAR-γ2 compared with E2-free adipogenic differentiation medium (p < 0.01 for LPL and p < 0.05 for PPAR-γ2).

RT-PCR analysis for adipogenic differentiation of ADSCs.
A: RT-PCR results of adipogenic differentiation of human ADSCs after exposure to a: normal culture medium, b: adipogenic differentiation medium, and c: adipogenic differentiation medium with E2 supplementation (10−8 M). B: Quantitative analysis of RT-PCR bands. Relative intensities were measured on RT-PCR bands by using ImageJ software. Student’s t-tests were used to indicate statistical significance. *p < 0.05; **p < 0.01.
The flow cytometry results are shown in Fig. 4A. Subsequent statistical analysis indicated that the early apoptosis rate of human ADSCs in the control, serum-free and serum-free + E2 groups was 0.4 ± 0.3%, 6.2 ± 0.4% and 0.8 ± 0.2%, respectively (Fig. 4B).

Flow cytometric analysis of ADSCs.
A: Three panels show human ADSCs in control culture medium (serum-containing), a serum-free environment (standard culture medium without serum) and a serum-free + E2 environment (standard culture medium without serum but supplement with E2), respectively. Viable cells show Annexin V−/PI− ; cells under early apoptotic phase show Annexin V+/PI−; cells under late apoptotic phase show Annexin V+/PI+; and necrotic cells show Annexin V−/ PI+. B: the apoptosis rate was determined as the ratio of the number of Annexin V+/PI cells over the total number of cells. Student’s t-tests were used to indicate statistical significance. **p < 0.01.
All the 21 nude mice that received fat transplantation were alive after 12 weeks. In total, 63 fat grafts were collected and the volume was measured. The control group (culture medium only) exhibited a survival ratio of 55.5 ± 1.5%. The use of human ADSCs significantly enhanced the survival ratio to 71.2 ± 1.7% (n = 21, p < 0.001). E2-treated human ADSCs had a more pronounced beneficial effect on the survival ratio (76.9 ± 1.9%), as shown in Fig. 5B.
Histological sections were taken from the center of each harvested tissue, and the central neo-vascularization was evaluated. As shown in Fig. 6A, the tissues harvested from the E2-treated human ADSC group showed more capillary production (22.4 per field of view) with a larger diameter than the untreated ADSC group and the control group, which showed obvious fibrosis (Fig. 6B).

Fat tissue transplantation in nude mice.
A: Surviving fat tissue after transplantation in a nude mouse (left) and extracted fat tissues (right). Labels a, b and c represent the use of E2-treated human ADSCs, untreated ADSCs and culture medium only, respectively, for fat transplantations. B: Survival ratio of transplanted fat tissues. Student’s t-tests were used to indicate statistical significance. *p < 0.05; ***p < 0.001.
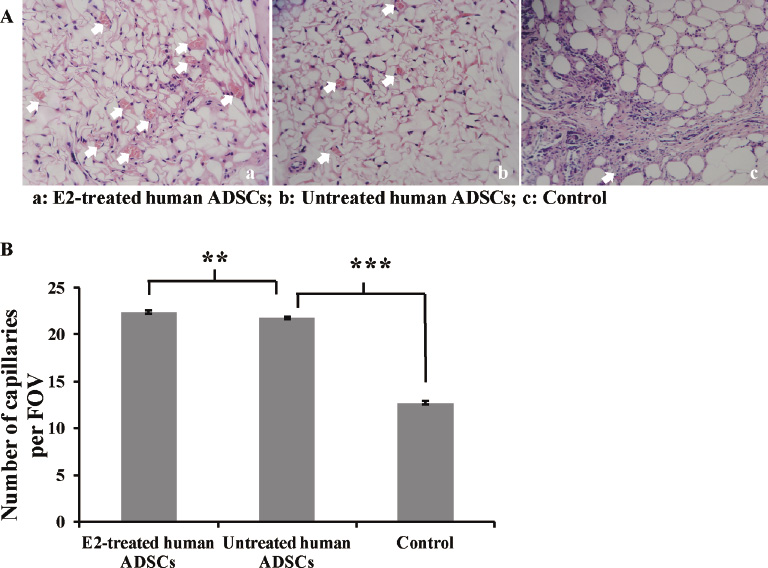
Histological analysis of transplanted fat tissues.
A: Histological evaluation of central sections from fat tissues 12 weeks after transplantation (100× magnification). Human ADSCs after treatment with E2 (a) resulted in more capillaries (marked by arrowheads) with a larger diameter than the untreated human ADSCs (b) and that treated with culture medium only (c), respectively. B: Capillary density in surviving fat tissues. The number of capillaries was counted from 10 fields per section taken from a central tissue section. Results are presented as the number of capillaries per field of view (FOV). Student’s t-tests were used to indicate statistical significance. **p < 0.01; ***p < 0.001.
Fat transplantation has shown great potential in plastic surgery and tissue repair due to its simple procedure and minimal donor-site morbidity (Xu et al. 2007; Lu et al. 2009). However, the clinical successes are greatly suppressed by the low survival ratio due to fat absorption and fibrosis (Lu et al. 2009). Previous studies have demonstrated that normal fat transplantation possesses a tissue reduction of 40-60% (Peer 1950; Chajchir et al. 1990). Recently, increased VEGF secretion showed a beneficial effect on tissue revascularization, with a subsequent enhancement in the survival ratio after transplantation (Li et al. 2000; Lu et al. 2009). Zhang et al. (2001) demonstrated that the preoperative treatment with VEGF induced angiogenesis and enhanced skin paddle survival. In addition, the use of stem cell tissue engineering has emerged as an efficient approach for various therapeutic purposes due to the ability of self-renewal and multiple differentiation into osteogenic, adipogenic, myogenic, chondrogenic and other cell lineages (Cancedda et al. 2003; Tuan 2004; Mauney et al. 2005). MSCs have been widely studied and show great efficiency in clinical applications (Hong et al. 2006, 2011). Previous studies have demonstrated that MSCs are able to produce various growth factors such as VEGF (Crisostomo et al. 2007) and hepatocyte growth factor (Wang et al. 2006) in vitro. These growth factors may promote angiogenesis (Wang et al. 2006), decrease production of proinflammatory cytokines (Yang et al. 2004, 2006) and reduce apoptosis (Ayala et al. 1996, 1998). However, MSC isolation from bone marrow remains a huge challenge and impedes their further development. A complicated, time-consuming and expensive surgical operation is required, and the quantity of isolated MSCs is limited. Recently, human ADSCs have been shown to exhibit similar differentiation, proliferation and VEGF secretion abilities as MSCs (Zuk et al. 2001; Cowan et al. 2004; Hong et al. 2007). Moreover, the abundant resource of ADSCs in the human body and the minimally invasive extraction procedure facilitate their superiority over MSCs (Hong et al. 2007). In this study, human ADSCs were extracted from different female patients through thigh liposuction. Each operation took a few minutes, with minimal pain or invasive injuries when compared to bone marrow aspiration, which require days-to-weeks for preparation, operation and rehabilitation. The isolated cells displayed characteristic cytometric expressions of ADSCs, and these results are in agreement with previous a study (Lu et al. 2009), indicating the efficiency of the extraction and isolation procedure of ADSCs.
The success of ADSCs-based fat transplantation relies on their proliferation and adipogenic differentiation abilities (Hong et al. 2007; Lu et al. 2009). Various studies have shown that the capacities of proliferation and adipogenic differentiation are lower than those of bone marrow MSCs (Hong et al. 2007), which remain a challenge for researchers to seek an effective solution.
E2, an important female sex hormone, plays a significant role in influencing growth, proliferation and differentiation of stem cells (Ramalho et al. 2001; Hong et al. 2004). In this study, the presence of E2 in the culture medium had a beneficial effect on human ADSC proliferation in vitro. Hong et al. demonstrated that E2 supplementation at a concentration of 10−8 M showed a more pronounced effect on proliferation activity than smaller concentration (10−9 M and 10−10 M) (Hong et al. 2007). Therefore, we hypothesized that increasing concentrations of E2 might further promote ADSC proliferation. Unfortunately, a 100-fold enhancement in E2 concentration (10−6 M) showed negligible effect on ADSC proliferation. However, results from the ELISA revealed that an E2 concentration of 10−8 M induced high VEGF production. These data indicate that E2 supplementation at a concentration of 10−8 M is the optimal condition for ADSC proliferation and VEGF secretion. VEGF production was further examined using a HUVEC model. We found that HUVEC proliferation (O.D. 490 nm) was correlated in an almost linear pattern to VEGF concentration. The actual mechanism of enhancement of proliferation and growth factor production induced by the presence of E2 is still under investigation. A possible explanation is that the interaction between E2 and estrogen receptor plays an important role in regulating ADSC behaviors (Qu et al. 1998; Oreffo et al. 1999; Yeh et al. 1999; Zhou et al. 2001).
The adipogenic differentiation of ADSCs is of great significance in controlling in vivo adipose tissue regeneration after transplantation. In this study, we further characterized the adipogenic differentiation behavior of ADSCs upon E2 supplementation. Differentiated adipocytes were stained by Oil-O-Red, and E2 supplementation at a concentration of 10−8 M showed a small but significant increase in the percentage of positively stained cells. RT-PCR and the following quantitative analysis also revealed that E2 supplementation with a concentration of 10−8 M showed beneficial gene expression changes when compared to the control. These results are in agreement with previous study (Hong et al. 2007).
The survival ratio is an important evaluation criterion in adipose transplantation. Traditional adipose transplantation often exhibits low survival ratio (~ 50%) (Chajchir et al. 1990). In this study, direct adipose injection (control group) resulted in an average survival ratio of 55.5%. Previous studies have suggested that the low survival ratio is mainly attributed to the fact that vascularization only occurs 48 hours post-transplantation (Fawcett 1948). This time-delayed vascularization causes limited survival of adipocytes due to damage of the nuclei and cell membranes (Lu et al. 2009). In this study, we investigated the apoptosis rate of the cells in a serum-free environment to simulate the early stage upon transplantation with low blood and oxygen supply. The results demonstrated that the apoptosis rate of human ADSCs (6.2 ± 0.4%) in a serum-free condition was significantly higher than that in the control group (p < 0.01). In contrast, the addition of E2 (10−8 M) had a beneficial effect in reducing the apoptosis rate, and no significant difference was found between the serum-free + E2 and control groups.
In one study, cell destruction due to lack of vascularization eventually resulted in adipose absorption (Ogawa 2006). Therefore, rapid and adequate vascularization is of great significance in the efficacy of adipose transplantation. Thus, in this study a series of ADSC-based lipoinjections were performed in a nude mice model to assess the efficacy of using ADSCs. Adipose tissue can be considered as a template substrate supporting ADSC growth and functionality (Ji and Shi 2012). In this study, ADSC-based transplantation resulted in a significantly higher survival ratio (71.2 ± 1.7%, p < 0.001) than the control group. Moreover, the exposure to E2 further enhanced the survival ratio (76.9 ± 1.9%). Histological examination also demonstrated enhanced capillary formation in the E2-treated ADSCs group when compared to the untreated ADSC and control groups. These results were in agreement with our in vitro studies showing that E2-treated ADSCs could produce more VEGF. Indeed, our results indicated that E2-supplemented culture medium induced a higher concentration of VEGF in ADSCs vitro, and upon transplantation, the presence of enhanced VEGF secretion promoted an increased capillary density and adipose viability.
Taken together, the use of E2-treaetd ADSCs for adipose transplantation holds great potential. Prior to clinical applications, longer-term animal studies are necessary to demonstrate the stability of adipose tissue. It is also worthwhile to note that the ADSCs used in this study were all obtained from female subjects. The effect of E2 on ADSCs isolated from male patients is still unclear and requires further investigations.
ADSCs were successfully extracted and isolated from four female outpatients using a simple liposuction method. The isolated human ADSCs demonstrated enhanced proliferation and VEGF secretion upon E2 supplementation in culture medium at an optimal concentration of 10−8 M. The addition of E2 showed beneficial effects in enhancing adipogenic differentiation of human ADSCs in vitro, as well as reducing the apoptosis of human ADSCs in a serum-free environment. The in vivo efficacy of human ADSCs-based fat transplantation was conducted in a nude mice model. E2 supplementation resulted in a higher survival ratio and more capillary formation. In summary, human ADSCs showed great potential in fat transplantation for plastic surgery and tissue repair.
This study was financially supported by the fund of clinical application of stem cells of Heilongjiang province. The authors would like to acknowledge Harbin Veterinary Research Institution of Chinese Academy of Agricultural Sciences for providing nude mice. The authors would like to acknowledge the 4th Affiliated Hospital of Medical University for providing HUVECs.
The authors declare no conflict of interest.