2013 Volume 231 Issue 2 Pages 145-158
2013 Volume 231 Issue 2 Pages 145-158
Bisphosphonates (BPs) are pyrophosphate analogs. They are widely used against enhanced bone-resorption in various diseases. Nitrogen-containing BPs (N-BPs) exhibit strong anti-bone-resorptive effects but have inflammatory and necrotic side effects. The non-nitrogen-containing BPs (non-N-BPs) etidronate and clodronate lack such side effects, but their anti-bone-resorptive effects are weak. In mice, etidronate and clodronate reduce the inflammatory/necrotic effects of N-BPs, even those of zoledronate, the N-BP with the strongest anti-bone-resorptive effect yet reported and the highest risk of inflammation/necrosis. Here, to explore the mechanisms underlying this protection, we used a mouse model in which a single reagent or a mixture of two reagents was injected subcutaneously into ear-pinnas. These reagents included zoledronate, four non-N-BPs, pyrophosphate, and inhibitors of various organic-anion-transporters. Pyrophosphate and two of the four non-N-BPs (not etidronate or clodronate) had inflammatory/necrotic effects. These effects were reduced by etidronate and clodronate, but not by phosphonoformate, an inhibitor of two of the three known phosphate-transporter families. Phosphonoformate reduced the inflammatory/necrotic effects of zoledronate, but not those of pyrophosphate or of non-N-BPs. Conversely, pyrophosphate, at non-inflammatory/necrotic concentrations, reduced the inflammatory/necrotic effects of non-N-BPs, but not those of zoledronate. The efficacies of the protective effects against the inflammatory/necrotic effects of zoledronate were clodronate > etidronate > phosphonoformate. These findings suggest that (i) the N-BP zoledronate may enter soft-tissue cells via phosphonoformate-inhibitable phosphate-transporters, (ii) other phosphate-transporters may carry pyrophosphate and inflammatory/necrotic non-N-BPs into such cells, and (iii) etidronate and clodronate inhibit all these transporters, and they ameliorate the side effects of zoledronate by inhibiting phosphonoformate-inhibitable phosphate-transporters.
Bisphosphonates (BPs) are widely used for diseases in which there is an enhanced bone-resorption because some have powerful anti-bone-resorptive effects (Rogers et al. 2011). BPs bind strongly to bone hydroxyapatite, and thus they accumulate within bone after repeated administration (Geddes et al. 2005). During bone-resorption, BPs are incorporated into osteoclasts and exhibit cytotoxicity (Rogers et al. 2011). There are two types of BPs, the nitrogen-containing BPs (N-BPs) and the non-nitrogen-containing BPs (non-N-BPs) (Fig. 1). Of these, the N-BPs have much more powerful anti-bone-resorptive effects than non-N-BPs, and for that reason the clinical use of N-BPs is greater than that of non-N-BPs. The cytotoxic effect of N-BPs on osteoclasts is due to intracellular inhibition of farnesyl pyrophosphate synthase, which is involved in the mevalonate pathway of cholesterol biosynthesis (Roelofs et al. 2006; Rogers et al. 2011). On the other hand, non-N-BPs are converted directly into cytotoxic ATP-analogs within cells (Klein et al. 1988; Rogers et al. 1996, 2011; Frith et al. 2001; Roelofs et al. 2006; Räikkönen et al. 2009).
It should be noted that the mevalonate pathway exists widely in eukaryotic cells. Thus, if an N-BP has the ability to enter cells, it may exhibit cytotoxicity in a wide variety of cells. Indeed, N-BPs, especially when given intravenously, have various inflammatory side effects, including fever, myelitis, and osteonecrosis of jawbones (ONJ, which may lead to exposure of the jawbones) (Ruggiero et al. 2004, 2009; Marx et al. 2005; Woo et al. 2006; Ruggiero 2009; Urade 2010; Pazinas and Abrahamsen 2011). N-BPs are also associated with ONJ when they are taken orally (though its incidence is much less than with intravenous administration of N-BPs) (Sedghizadeh et al. 2009), and their oral use entails a relatively high risk of upper gastrointestinal injuries (Anastasilakis et al. 2007). A notable point is that there are fewer reports of inflammatory/necrotic side effects in patients treated with non-N-BPs (Crépin et al. 2010) despite them having been in use for a much longer time than N-BPs (e.g., etidronate was approved in 1977 in the USA and in 1990 in Japan).
In mice, all the N-BPs tested so far exhibit inflammatory and/or necrotic effects, such as induction of the key enzyme in histamine formation, increased granulocytic cells, hematopoiesis, and interleukin-1 (IL-1) production (Endo et al. 1993, 1999: Sugawara et al. 1998; Nakamura et al. 1999). When injected topically, N-BPs induce inflammation and/or necrosis at the injection site (Schenk et al. 1986; Oizumi et al. 2009). An inhibition of the intracellular mevalonate pathway within soft tissues has been suggested to be causally involved in the inflammatory and necrotic effects of N-BPs in soft tissues in mice (Deng et al. 2007; Marcuzzi et al. 2008). If that is so, N-BPs may display their inflammatory and/or necrotic effects after being taken up by cells within soft tissues. Indeed, 14C-pamidronate (an N-BP) is retained for a few days within soft tissues after its intravenous injection into mice (Mönkkönen et al. 1989). However, exactly how N-BPs enter soft-tissue cells remains uncertain. Interestingly, etidronate and clodronate (both non-N-BPs) are hardly retained at all within soft tissues (Mönkkönen et al. 1989), and they do not exert such inflammatory and necrotic effects in mice. Moreover, they reduce the amounts of zoledronate and alendronate (both N-BPs) retained within soft tissues such as liver, spleen, lung, and ear-pinna in mice (Oizumi et al. 2009, 2010), and they also reduce or prevent the inflammatory and necrotic effects of N-BPs (Table 1). These findings suggest that etidronate and clodronate antagonize the entry of N-BPs into soft-tissue cells. However, the literature lacks studies on the in vivo entry of N-BPs into soft-tissue cells, although there are reports of them being taken up into osteoclasts via fluid-phase endocytosis under an acidic environment (Thompson et al. 2006; Coxon et al. 2008; Rogers et al. 2011).
Here, we set out to clarify the mechanisms underlying the protective effects of etidronate and clodronate described above, and also to determine the characteristics of the in vivo cellular entry of N-BPs. To those ends, we examined the antagonisms among various BPs and BP-related substances when they were co-injected into mouse ear-pinnas. We used zoledronate as an N-BP because this drug has the most powerful anti-bone-resorptive effect yet reported and the highest risk of inflammatory and necrotic side effects in both humans and mice (Oizumi et al. 2009, 2010). We also tested several inorganic or organic anions with a structural similarity to BP, including pyrophosphate (PPi) and phosphonoformate (PFA) (Fig. 1), in experiments designed to examine their abilities to reduce the adverse effects induced by BPs. PFA is known to be an inhibitor of the phosphate-transporter families SLC34 and SLC20, although it inhibits the latter only at higher concentrations (Virkki et al. 2007).

Structures of substances tested in the present study. Bisphosphonates (BPs) with a non-hydrolysable P-C-P structure are the analogs of pyrophosphate (PPi), which has a hydrolysable P-O-P structure. There are two types of BPs, the nitrogen-containing BPs (N-BPs) and the non-nitrogen-containing BPs (non-N-BPs). Etidronate (Eti), clodronate (Clo), medronate (Med), and oxidronate (Oxi) are non-N-BPs, while zoledronate (Zol) is an N-BP. Phosphonoformate (PFA) is an inhibitor of the phosphate transporter SLC34 (at higher concentrations it also inhibits SLC20) (Virkki et al. 2007). The relative potencies of their anti-bone-resorptive effects (ABREs) are also shown (those of oxidronate and medronate being determined in the present study). #Inflammatory and necrotic substances (see text). *Relative ABREs determined in this study (see text).

Effects of etidronate (Eti) and clodronate (Clo) on the inflammatory/necrotic effects (INSEs) and anti-bone-resorptive effects (ABREs) of N-BPs.
aEti and Clo (both non-N-BPs, the first generation of BPs) have almost no INSEs. However, they antagonize the INSEs of various N-BPs in mice (Endo et al. 1999; Funayama et al. 2005; Oizumi et al. 2009, 2010) possibly by reducing the amount of N-BPs retained within soft tissues (Oizumi et al. 2009, 2010). For these antagonizing effects, Clo > Eti.
bEti reduces the ABREs of N-BPs by antagonizing their binding to bone hydroxyapatite (Funayama et al. 2005; Oizumi et al. 2010).
cThe ABRE of Clo + an N-BP is similar to or greater than that of the same N-BP alone (Monma et al. 2004). This may be due to (i) the affinity of Clo for bone hydroxyapatite being less than that of Eti and/or (ii) the ABRE of Clo being greater than that of Eti.
dEti (at a larger dose than used clinically), even when injected after an N-BP injection, can remove bone-bound N-BPs (Oizumi et al. 2010).
eOn the basis of these findings, we have proposed that Eti and/or Clo may be utilized as a substitution drug for N-BPs and/or as a combination drug with a N-BP (Monma et al. 2004; Funayama et al. 2005; Yamaguchi et al. 2010; Oizumi et al. 2009, 2010).
BALB/c mice were bred in our laboratory. All experiments complied with the Guidelines for Care and Use of Laboratory Animals in Tohoku University. Zoledronate was from Toronto Research Chemicals Inc. (North York, ON, Canada). Medronate and clodronate were from Sigma (St. Louis, MO, USA). Oxidronate was synthesized to order (Quimby et al. 1967). Etidronate, PPi, PFA, amino acids, and other compounds were purchased from Wako Pure Chemical Industries (Osaka, Japan). The above drugs were dissolved in sterile saline, with the pH of the solutions being adjusted to 7 with NaOH or HCl. Experimental protocols are described in the text or in the legend to the Figure relating to each experiment.
Inflammatory and necrotic effects of BPsFemale mice (6-8 weeks of age) were anesthetized with ethyl ether, and a BP solution was injected subcutaneously into both the right and the left pinna (inside) near the root of the ear (20 µl each ear) (3-8 mice/group). The concentrations used are indicated in the relevant experiments. As described below, the inflammatory and necrotic actions of BPs were evaluated daily (Oizumi et al. 2009). All experiments were terminated on day 7.
(a) Inflammation: The length (L) and width (W) of the area of inflammation at the back of the ear (detectable as a red area) were recorded, and L × W (mm2) was used as an indicator of inflammation.
(b) Necrosis: After maximum inflammation (estimated as described above) had been attained, the center of the inflammatory site became necrotic, detectable as a change of color from red to dark brown (or black) or as a tissue defect. At the start of the necrosis, we stopped measuring inflammation. In each group of mice, we recorded the number of ears with necrosis and the number without necrosis, then calculated the incidence of necrosis (e.g., maximum incidence is 8 in a group of 4 mice).
Estimation of the anti-bone-resorptive effects of BPsA clear sclerotic band (which a few years ago we tentatively called the BP-band) is detectable in tibias by radiography a few weeks after a single intraperitoneal injection of a BP into mice, reflecting an inhibition of bone resorption (Monma et al. 2004; Funayama et al. 2005; Yu et al. 2005). Hence, we can estimate the anti-bone-resorptive effects of BPs by using the BP-band as a marker. Briefly, each BP solution was intraperitoneally injected (0.1 ml/10 g body weight) into young male mice (4-5 weeks old). The mice were decapitated two weeks later, and tibias were removed and subjected to X-ray analysis for the detection and quantification of the BP-bands. To this end, Soft X-ray radiophotographs were taken using SOFTEX and Fuji Industrial X-ray film, the conditions being 80 V, 1 mA, duration 55 sec (Monma et al. 2004), and the BP-bands were quantitatively analyzed using NIH Image software. In this analysis, we recorded a value derived by multiplying the “mean gray value” (average gray value of pixels within a selected band) by the area (mm2) of that BP-band. In each experiment, a value obtained from a corresponding area of a control tibia excised from a mouse given no BP was subtracted from the above experimental value.
Data analysisExperimental values are given as mean ± standard deviation (s.d.). The statistical significance of the difference between two means was evaluated using a Bonferroni multiple-comparison test. For differences in incidence between 2 experimental groups at a given time, analysis was by the Fisher exact probability test. P values less than 0.05 were considered to be significant.
There is a lack of reports comparing relative potencies among etidronate, clodronate, oxidronate, and medronate. As described in Methods, we can estimate the anti-bone-resorptive effects of BPs by measuring the BP-band that appears in tibias after a single injection of a BP. Thus, we examined (i) the relative abilities of the four non-N-BPs to produce a BP-band and (ii) the modulating effects of those four non-N-BPs on the induction of a BP-band by zoledronate.
(1) Anti-bone-resorptive effects of non-N-BPs: As shown in Fig. 2A, intraperitoneal injection of any one of the four non-N-BPs tested, at 10 mM, resulted in the formation of a BP-band in the tibia in young mice. The relative potencies for BP-band formation were oxidronate ≥ clodronate etidronate ≈ medronate.
(2) Effects of non-N-BPs on the anti-bone-resorptive effect of zoledronate: Zoledronate is known to have the most powerful anti-bone-resorptive effect among the BPs in current clinical use. As shown in Fig. 2B, zoledronate formed a clear BP-band even at 0.005 mM. The ability of zoledronate to form this clear BP-band was markedly reduced by its combined administration with etidronate or medronate. On the other hand, despite zoledronate, oxidronate, and clodronate each having the ability to form a clear-BP band at the doses used in this experiment, the abilities of zoledronate + oxidronate and zoledronate + clodronate to form BP-bands were similar to that of zoledronate alone. These results suggest that (i) there is an antagonism between zoledronate and a given non-N-BP regarding binding to bone, and (ii) the anti-bone-resorptive effect of a combination zoledronate + a given non-N-BP is determined by the amounts of the two agents that become bound to bone.
Incidentally, it was an important finding that the anti-bone-resorptive effects of zoledronate + clodronate and zoledronate + oxidronate are much greater than those of zoledronate + etidronate and zoledronate + medronate. The importance is outlined below when we consider the protective effects against the inflammatory and necrotic effects of zoledronate that may be obtained if such combinations are administered clinically.

Anti-bone-resorptive effects of BPs, alone or in combination. Anti-bone-resorptive effects were quantitatively evaluated on the basis of BP-band formation (see Materials and Methods). A representative BP-band is shown for each group, together with data from the quantitative analysis of the BP-bands. (A) Anti-bone-resorptive effects of the four non-N-BPs used in the present study. Oxidronate (Oxi), medronate (Med), clodronate (Clo), or etidronate (Eti) (each at 10 mM) was injected intraperitoneally into mice, and 2 weeks later tibias were subjected to analysis of BP-bands. n = 6. (B) Effects of non-N-BPs on the anti-bone-resorptive effects of Zol. Zol (0.005 mM), either alone or in a mixture with Oxi, Med, Clo, or Eti (each at 10 mM), was injected intraperitoneally into mice, and 2 weeks later tibias were subjected to analysis of BP-bands. n = 6.
Neither etidronate nor clodronate is inflammatory or induces necrosis when injected into ear-pinnas, even at 100 mM (Oizumi et al. 2009). Thus, we anticipated that oxidronate and medronate, which like etidronate and clodronate are non-N-BPs (Fig. 1), might not have inflammatory and necrotic effects. Surprisingly, however, oxidronate and medronate each induced inflammation at the site of injection (Fig. 3A). In addition, oxidronate had a necrotic effect, even at 5 mM. We have reported elsewhere that the rank order of potencies with which N-BPs induce inflammatory and necrotic effects in mice is zoledronate > pamidronate ≥ alendronate ≥ risedronate (Oizumi et al. 2009). Since necrotic effects were observed for zoledronate at ≥ 2 mM and for pamidronate at ≥ 16 mM in that study (Oizumi et al. 2009), and for oxidronate at ≥ 5 mM in the present study, the potency with which oxidronate induces its inflammatory and necrotic effects is less than that of zoledronate, but greater than that of pamidronate. Although a necrotic effect of medronate was not evident at 10 mM, it was evident at 100 mM (data not shown).
Oxidronate and medronate have molecular structures that are very similar to that of PPi (Fig. 1). We therefore examined whether PPi has inflammatory and/or necrotic effects. As shown in Fig. 3B, PPi was inflammatory at > 20 mM and necrotic at 50-100 mM. The similarity among PPi, oxidronate, and medronate in terms of molecular structure (Fig. 1) and the finding that all three have inflammatory and necrotic effects led us to suppose that they might enter cells via a common transporter(s).

Inflammatory and necrotic effects of oxidronate (Oxi), medronate (Med), and PPi. (A) Effects of Oxi and Med. After Oxi (5 or 10 mM) or Med (10 mM) had been injected subcutaneously into ear-pinnas (20 l each ear), the inflammatory areas and the incidence of necrosis were evaluated (see Materials and Methods). n = 8. Injection of saline induced no detectable inflammatory reactions (data not shown). (B) Effects of PPi. Various concentrations of PPi were injected subcutaneously into ear-pinnas (20 µl each ear), and the inflammatory areas and the incidence of necrosis were evaluated (see Materials and Methods). n = 8.
As reported previously, etidronate and clodronate can each reduce or prevent the inflammatory and necrotic effects of N-BPs, clodronate being more potent in this respect than etidronate (Oizumi et al. 2009, 2010). However, the non-N-BP oxidronate surprisingly induced potent inflammatory and necrotic effects as described above. We therefore examined whether etidronate and clodronate might reduce the inflammatory and necrotic effects of oxidronate. As shown in Fig. 4A, etidronate (50 mM) reduced the necrotic effect of oxidronate, although it did not completely abolish it. On the other hand, clodronate strongly reduced both the inflammatory and necrotic effects of oxidronate, with 50 mM clodronate completely preventing oxidronate’s necrotic effect (Fig. 4B). Incidentally, data not shown here revealed that (i) medronate (10 mM) had no detectable effect on either the inflammation or necrosis induced by 10 mM oxidronate, and (ii) etidronate and clodronate (each at 50 mM) reduced the necrotic effect of medronate (100 mM). These results indicate (a) that etidronate and clodronate can each reduce or prevent the inflammatory and necrotic effects of oxidronate and medronate, and (b) that as regards this protective effect, clodronate is more potent than etidronate. Notably, clodronate is also more potent than etidronate as regards its reducing effect on the inflammatory and necrotic effects of N-BPs [Table 1, footnote (a)].

Effects of etidronate (Eti) and clodronate (Clo) on the inflammatory and necrotic effects of oxidronate (Oxi). After Oxi (10 mM), either alone or in a mixture with Eti or Clo (each at 10 or 50 mM), had been injected subcutaneously into ear-pinnas (20 µl each ear), the inflammatory areas and the incidence of necrosis were evaluated (see Materials and Methods). n = 8.
We first examined whether PPi might antagonize the inflammatory and necrotic effects of oxidronate. As shown in Fig. 5A, PPi at 10 mM reduced the inflammatory and necrotic effects of oxidronate. We also examined the effect of PPi on the inflammatory and necrotic effects of medronate (data not shown). Medronate was necrotic at 100 mM, and PPi (5 and 10 mM) reduced or tended to reduce this necrotic effect of medronate. However, higher concentrations of PPi (20 and 50 mM) were not effective at reducing the inflammatory and necrotic effects of medronate. On the other hand, PPi (10 mM) did not reduce the inflammatory and necrotic effects of zoledronate (indeed, it tended to augment the latter’s necrotic effect) (Fig. 5B). These results suggest that PPi can antagonize the inflammatory and necrotic effects of both oxidronate and medronate, but that when PPi is given at higher concentrations, its own inflammatory and necrotic effects may mask its protective effects against the inflammatory and necrotic effects of oxidronate and medronate. The above results also suggest that PPi does not antagonize the inflammatory and necrotic effects of zoledronate.
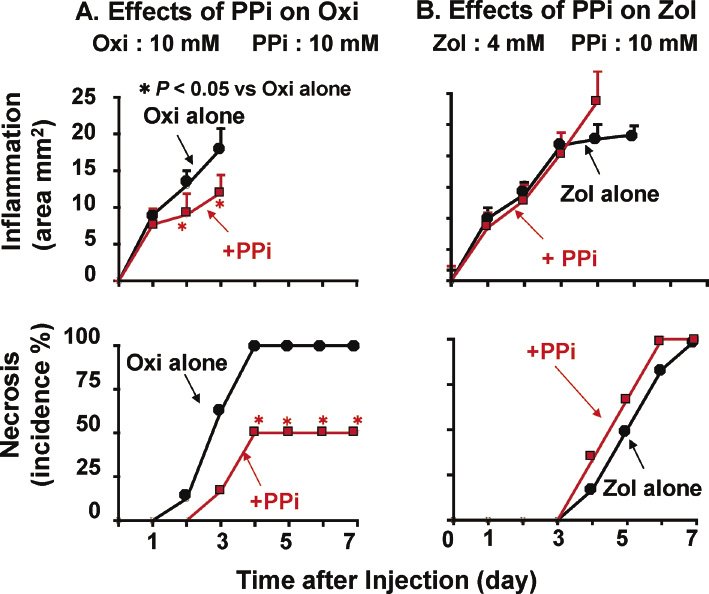
Effects of PPi on the inflammatory and necrotic effects of oxidronate (Oxi) and zoledronate (Zol). After Oxi (10 mM) or Zol (4 mM), either alone or in a mixture with PPi (2.5, 5, 10, or 20 mM), had been injected subcutaneously into ear-pinnas (20 µl each ear), the inflammatory areas and the incidence of necrosis were evaluated (see Materials and Methods). n = 16 (A), n = 8 (B).
We examined the effects of etidronate and clodronate on the inflammatory and necrotic effects of PPi (50 or 100 mM). As shown in Fig. 6A and B, etidronate and clodronate each reduced or prevented the inflammatory and necrotic effects induced by both 50 and 100 mM PPi. In this case, too, the protective effect of clodronate was more potent than that of etidronate.

Effects of etidronate (Eti) and clodronate (Clo) on the inflammatory and necrotic effects of PPi. After PPi (50 or 100 mM), either alone or in a mixture with Eti or Clo (each 50 mM), had been injected subcutaneously into ear-pinnas (20 µl each ear), the inflammatory areas and the incidence of necrosis were evaluated. n = 8.
Non-N-BPs have two acidic (phosphate) residues, while N-BPs have one nitrogen-containing residue and two acidic (phosphate) residues. We hypothesized that some natural molecules with two acidic (carboxyl) residues or with two acidic residues and one nitrogen-containing residue might antagonize the inflammatory and necrotic effects of non-N-BPs or N-BPs. To test this idea, we examined the effects of various molecules (including malonate, succinate, lactate, aspartate, glutamate, and histidine) against the inflammatory and necrotic effects of zoledronate and oxidronate. Although statistical significance was not reached, malonate (10 mM) tended to reduce the inflammatory and necrotic effects of oxidronate (5 and 10 mM), while aspartate and glutamate tended to reduce the inflammatory and necrotic effects of zoledronate (2 mM) (data not shown). However, TFB-TBA (a specific inhibitor of glutamate transporters) (Shimamoto et al. 2004) was not effective.
Since many drugs are known to be incorporated into cells via SLC-transporters, the effects of the following drugs on the inflammatory and necrotic effects of zoledronate were examined (the relevant transporters are shown in parentheses): cimetidine [SLC22 (A2) and SLC47 (A1 and A2)], probenecid [SLC22 (A6 and A8)], quinidine [SLC22 (A1 and A2), SLC47 (A1 and A2), and ABC (B1)], cyclosporine [SLCO1 (B1 and B3) and SLCO2 (B1) and ABC (B1, B4, B11, and C2), and riphampicin [SLCO1 (B1 and B3) and SLCO2 (B1)] (The International Transporter Consortium 2010). The SLC22 family transports organic anions. SLCO1 also transports organic anions (including various drugs), while SLCO2 transports prostaglandins and steroids (He et al. 2009). However, none of the above substances was effective at reducing the inflammatory or necrotic effects of zoledronate (data not shown). These results lead us to speculate that the transporters described above might be of little or no importance in the cellular uptake of zoledronate.
Effects of PFA, an inhibitor of phosphate transportersAs described above, like etidronate and clodronate, PPi reduced the inflammatory and necrotic effects of oxidronate (Fig. 5A), while etidronate and clodronate reduced the inflammatory and necrotic effects of N-BPs, oxidronate, and PPi (Tables 1 and 2). These findings and the negative results described in the preceding section led us to suppose that phosphate transporters might be involved in the intracellular uptake of N-BPs and non-N-BPs with inflammatory and necrotic effects. In the literature, we found evidence that PFA, etidronate, and clodronate inhibit phosphate transporters (Szczepanska-Konkel et al. 1986; Ullrich et al. 1997). Hence, we tested the effect of PFA on the inflammatory and necrotic effects of zoledronate, oxidronate, and PPi. By itself, PFA had no detectable inflammatory and necrotic effects at 100 mM (data not shown). As shown in Fig. 7A, the inflammatory and necrotic effects of zoledronate were reduced by PFA in a dose-dependent manner, with 20 and 50 mM PFA completely preventing the necrosis induced by zoledronate (2 mM). However, the inflammatory and necrotic effects of oxidronate (10 mM) and PPi (50 mM) were not reduced by PFA, even at 50 mM (Fig. 7B and C).

Summary of the effects of etidronate (Eti), clodronate (Clo), PPi (low concentrations), and PFA on the inflammatory and necrotic side effects (INSEs) of zoledronate (Zol), oxidronate (Oxi), and of high concentrations of PPi.
aThe protective effects of Clo against the INSEs of Zol, Oxi (or Med), and PPi were greater than those of Eti.
bClo at 4 mM completely prevents the necrotic effect of 4 mM Zol (Oizumi et al., 2009). The necrotic effect of 2 mM Zol is completely prevented by 8 mM Eti (Oizumi et al., 2010) and by 20 mM (but not 10 mM) PFA (Fig. 7A). Thus, the relative potencies of the protective effects against the inflammatory/necrotic effects of Zol are Clo > Eti > PFA.
It should be noted that the following substances exhibited no significant protective effects. (i) Substances with two carboxyl residues or one additional nitrogen-containing residue: malonate, succinate, lactate, aspartate, glutamate, and histidine. (ii) Known inhibitors of transporters [the name(s) of the transporter(s) are given within brackets]: TFB-TBA (glutamate transporter), cimetidine [SLC22 (A2) and SLC47 (A1 and A2)], probenecid [SLC22 (A6 and A8)], quinidine [SLC22 (A1 and A2), SLC47 (A1 and A2), and ABC (B1)], cyclosporine [SLCO1 (B1 and B3) and SLCO2 (B1) and ABC (B1, B4, B11, and C2), and riphampicin [SLCO1 (B1 and B3) and SLCO2 (B1)].

Effects of PFA on the inflammatory and necrotic effects of zoledronate (Zol), oxidronate (Oxi), and PPi. After Zol (2 mM), Oxi (10 mM), or PPi (50 mM), either alone or in a mixture with PFA (5, 10, 20, or 50 mM), had been injected subcutaneously into ear-pinnas (20 µl each ear), the inflammatory areas and the incidence of necrosis were evaluated. n = 8.
The effects of etidronate, clodronate, PPi (low concentrations), and PFA on the inflammatory and necrotic effects of zoledronate, oxidronate, and PPi (high concentrations) in mouse ear-pinnas are summarized in Table 2. We found that the anti-bone-resorptive effects of zoledronate + clodronate and zoledronate + oxidronate were much greater than those of zoledronate + etidronate and zoledronate + medronate. These findings, together with those made in previous studies, are discussed in the following paragraphs.
Inflammatory/necrotic effects of zoledronate in mouse ear-pinnasN-BPs, when taken orally by human patients, carry the risk of direct injury to esophageal and gastrointestinal epithelial tissues (Adami and Zamberlan 1996). In mice and in human patients, N-BPs (as well as non-N-BPs) accumulate within bones (Geddes et al. 2005; Oizumi et al. 2010), especially in those bones exhibiting inflammation (Yamaguchi et al. 2010). Thus, it is likely that once N-BPs have accumulated within jawbones, they are released from the bone during bone destruction or as a result of injuries caused by tooth extraction and/or infection. This release from bone would include a release from osteoclasts that have been killed by the N-BP. This idea of release from bone may be supported by the finding of Scheper et al. (2009) that zoledronate can be detected in the saliva of zoledronate-treated patients. Conceivably, the released N-BP may directly injure the surrounding soft-tissue cells. In the present study, injection of N-BPs into mouse ear-pinnas directly induced inflammatory/necrotic effects at the site of injection. Thus, experiments like ours may be considered to be a convenient way of studying the inflammatory and/or necrotic profiles of N-BPs in soft tissues.
Inflammatory/necrotic effects of oxidronate and medronateAlthough they are non-N-BPs, oxidronate and medronate displayed inflammatory/necrotic effects, and the order of potencies for such effects is zoledronate > oxidronate > pamidronate ≥ alendronate ≥ risedronate > PPi ≥ medronate. In contrast, it is noteworthy that etidronate, clodronate, and PFA do not exhibit inflammatory/necrotic effects even at 100 mM (Oizumi et al. 2009, and the present study). It has been reported that non-N-BPs are converted to cytotoxic ATP-analogs within cells (Roelofs et al. 2006; Rogers et al. 2011). Thus, upon entry into cells, a non-N-BP may become cytotoxic, resulting in inflammation and/or necrosis of the tissue. Oxidronate is used clinically as a carrier of 99mTc for bone scintigraphy, and for that purpose it is given as a single intravenous injection at a dose of 0.4 mg or less. This dose is much lower than the intravenous doses of N-BPs given clinically (30-45 mg per patient for pamidronate, 20 mg for alendronate, and 4 mg for zoledronate). Thus, oxidronate would not be expected to induce any inflammatory/necrotic effects when it is used in the clinic in the above way.
Mechanism by which oxidronate and medronate may enter soft-tissue cellsOsteoclasts secrete HCl, which dissolves bone hydroxyapatite. Under such a strong acidic condition as that pertaining beneath osteoclasts, non-N-BPs (as well as N-BPs) are released from the dissolving bone hydroxyapatite and are then taken up by the osteoclasts. This is able to occur because in the acidic environment, binding of protons to phosphate residues reduces their polarity, thereby making them lipophilic and subject to passive transportation into osteoclasts. However, in a neutral environment (e.g., in most soft tissues), non-N-BPs are highly polarized by loss of their protons. In that environment, they scarcely enter cells at all across the cell membrane (Buxton 2006), except in the presence of a specific transport system. Recent studies have revealed that the solute-carrier (SLC) gene superfamily encodes membrane-bound transporters, comprising 55 gene families with at least 362 putatively functional protein-coding genes (He et al. 2009). Various ionic substances (including organic anions, amino acids, and various drugs) have been shown to be incorporated into cells via these SLC transporters (Giacomini and Sugiyama 2006; The International Transporter Consortium 2010; Anderson and Thwaites 2011). Among the SLC families, 3 families are known to be phosphate transporters (Virkki et al. 2007; He et al. 2009; Miyamoto et al. 2011). In view of (i) the molecular size, structural similarity (all of them are phosphate-containing substances), and polarity of non-N-BPs, and (ii) the finding that the inflammatory and necrotic effects of oxidronate and medronate are inhibited by PPi (a natural inorganic anion), we suggest that the xenobiotics oxidronate and medronate may be taken up into soft-tissue cells by “hitching a ride” upon a certain type of SLC phosphate transporter(s).
Mechanism by which zoledronate may enter soft-tissue cellsEtidronate and clodronate reduce the inflammatory and necrotic effects of various N-BPs, including zoledronate (Oizumi et al. 2009, 2010). The present experiments indicated that in addition, etidronate and clodronate could reduce the inflammatory and necrotic effects of the non-N-BPs oxidronate and medronate. In contrast, PPi did not reduce the inflammatory and necrotic effects of zoledronate, although it did reduce those of oxidronate and medronate. PFA, an inhibitor of the phosphate transporters SLC20 and/or SLC34 (Virkki et al. 2007), reduced the inflammatory and necrotic effects of zoledronate, but not those of oxidronate and PPi, indicating that the protective effect of PFA may be selective for the inflammatory/necrotic effects of N-BPs. We know of no evidence and no references indicating that PFA inhibits other transporters (such as SLC17, another family of phosphate transporters). Moreover, SLC20 transporters are known to be present ubiquitously throughout the body (Virkki et al. 2007; Miyamoto et al. 2011). Thus, the results described above suggest that (i) zoledronate may largely enter soft-tissue cells via SLC20 and/or SLC34, whereas (ii) oxidronate, medronate, and PPi may largely enter via another transporter(s), such as SLC17 (Fig. 8).
Our conclusions differ from that reached by Thompson et al. (2006) and also by Coxon et al. (2008), who suggested that N-BPs may be taken up by macrophages via fluid-phase endocytosis (a transport system for particles). One possibility is that this difference is somehow related to the experimental conditions, our studies being in vivo and the latter’s being in vitro. However, it is conceivable that SLC transporters convey N-BPs from endocytic vesicles into the cytosol. If so, both mechanisms may be in operation. Moreover, PFA (also known as “foscarnet”) has other activities, such as (i) anti-viral activity via an inhibition of viral DNA synthesis due to its interaction with viral DNA polymerase or reverse transcriptase (Chrisp and Clissold 1991) and (ii) inhibition of calcium deposition (Villa-Bellosta and Sorribas 2009). We cannot rule out contribution(s) by these effects or via as yet unknown mechanisms.

Schematic representation of the putative mechanisms discussed in the present report. Zoledronate (Zol) may enter soft-tissue cells via the phosphate transporter families SLC20 and/or SLC34, resulting in inhibition of cholesterol biosynthesis and increases in cytotoxic ATP-analogs, effects that underlie its inflammatory and necrotic side effects (INSEs). PPi and the non-N-BPs oxidronate (Oxi) and medronate (Med) may be transported via another family of phosphate transporters (viz. SLC17), the non-N-BPs each inducing an increase in cytotoxic ATP-analogs, effects that underlie their INSEs. Etidronate (Eti) and clodronate (Clo) (non-N-BPs that have almost no INSEs) inhibit all of these transporters, while PPi inhibits SLC17. These inhibitions protect against the INSEs of the following: Zol, the non-N-BPs Oxi and Med, and PPi. In contrast, PFA, a known inhibitor of SLC20 and/or SLC34, protects against the INSEs of Zol, but not against those of Oxi, Med, and PPi. The SLC17, SLC20, and SLC34 families are reported to include 9, 2, and 3 members, respectively (He et al. 2009; Omote et al. 2011).
Frith and Rogers (2003) reported that (i) clodronate potently antagonized the in vitro effects of N-BPs in both osteoclasts and J774 cells (a macrophage cell line), and (ii) clodronate reduced the in vitro uptake of 14C-ibandronate (an N-BP) by J774 cells. From these results, they supposed that competition exists between clodronate and ibandronate for cellular uptake by a membrane-bound transport protein. However, in spite of its potent inhibition of the in vitro effects of N-BPs, clodronate’s competitive effect against the uptake of 14C-ibandronate by J774 cells was very weak. Indeed, it reached statistical significance only after J774 cells had undergone a long period in culture (7 h or more) in the presence of 25 µM ibandronate and a 40-fold excess of clodronate (i.e., 1 mM). In contrast, in mouse ear-pinnas, clodronate affords complete protection against the necrotic effect of zoledronate when each agent is injected at 4 mM (Oizumi et al. 2009). Thus, we postulate that: (i) the in vitro antagonizing effect of clodronate is not due to competition for cellular uptake by a “specific” transporter protein, and (ii) the in vitro mechanism may differ from that observed in the present in vivo experiments.
Töyräs et al. (2003) reported that (i) alendronate (an N-BP) augmented the LPS-induced in vitro production of inflammatory cytokines (IL-1β, IL-6, and TNF-α) by RAW 264 macrophages, and (ii) the production of these cytokines was markedly inhibited by clodronate. Concerning this inhibitory effect of clodronate, they supposed that clodronate inhibits the binding of nuclear factor-κB (NF-κB) to DNA, because the cytotoxic ATP analog derived from the intracellular metabolism of clodronate inhibits the binding of NF-κB to DNA (Makkonen et al. 1999). However, as described in the next section, clodronate is hardly retained at all within soft tissues in vivo. Thus, it seems unlikely that the above effect occurred in the present in vivo experiments, even though it can be observed in vitro.
Mechanism underlying the in vivo protective effects of etidronate and clodronateIt should be noted that etidronate and clodronate display no detectable inflammatory and necrotic effects in mouse ear-pinnas even at 100 mM (Oizumi et al. 2009). 14C-etidronate and -clodronate are not retained within soft tissues in mice (i.e., they are rapidly excreted by the kidney and soon disappear from the blood), whereas 14C-pamidronate (an N-BP) is retained for several days within soft tissues such as liver and spleen (Mönkkönen et al. 1989). These results suggest that etidronate and clodronate hardly enter soft-tissue cells at all. We have shown elsewhere that etidronate and clodronate reduced or prevented the inflammatory and necrotic effects of all the N-BPs tested (alendronate, incadronate, ibandronate, and zoledronate), with the relative potencies of their protective effects being clodronate > etidronate (Endo et al. 1999; Funayama et al. 2005; Oizumi et al. 2009, 2010) (Table 1). As mentioned in the legend to Table 2, the rank order of potencies for protection against the inflammatory and necrotic effects of zoledronate is clodronate > etidronate > PFA. Moreover, etidronate and clodronate also reduced the inflammatory and necrotic effects of oxidronate, medronate, and PPi, with the relative potencies for these protective effects, again being clodronate > etidronate. It is notable that in structure, these four non-NBPs and PPi are very similar to each other (Fig. 1). It should be noted that by bioassay of N-BPs (including zoledronate and alendronate), we have previously shown that clodronate and etidronate reduce the amount of an N-BP retained within soft tissues (ear-pinnas, liver, lung, and spleen) (Oizumi et al. 2009, 2010) (Table 1). Our recent preliminary experiments indicate that clodronate and etidronate each reduce the amount of [2,3-3H]-alendronate (an N-BP) retained in mouse ear-pinnas, with the rank order of potencies being clodronate > etidronate > PFA (T. Kiyama et al., unpublished data). These results suggest that clodronate and etidronate inhibit the intracellular entry of N-BPs. It should also be noted that PFA, etidronate, and clodronate inhibit phosphate transporters (Szczepanska-Konkel et al. 1986; Ullrich et al. 1997), but they do not inhibit transporters for other anions, such as p-aminohippurate, sulfates, or dicarboxylates (Ullrich et al. 1997). At present, we cannot directly measure BPs within cells. However, the findings summarized in Tables 1 and 2, together with the structural similarity among etidronate, clodronate, PPi, PFA, and N-BPs (Fig. 1) (i.e., they are all phosphate-containing substances), strongly support the following ideas (i) etidronate and clodronate interact with all of the phosphate transporters (i.e., SLC17, SLC20, and SLC34), (ii) they thereby inhibit the entry of other phosphate-containing substances into soft-tissue cells, and (iii) etidronate and clodronate themselves may hardly enter soft-tissue cells at all. The above discussion may be simply summarized as illustrated in Fig. 8. However, we must emphasize that the scheme shown is tentative, and in this area much more research is needed. Such research should include studies into the possible contributions made by other transporters as well as an examination of the possibility that substances other than transporters are involved.
Effects of combined administration of an N-BP with etidronate or clodronateThe anti-bone-resorptive effects of BPs depend on their binding to bone hydroxyapatite and on their incorporation into osteoclasts, as well as on the intracellular potencies with which they inhibit farnesyl pyrophosphate synthase and/or produce cytotoxic ATP analogs (Roelofs et al. 2006; Rogers et al. 2011). As previously described in detail (Monma et al. 2004; Funayama et al. 2005; Yu et al. 2005; Oizumi et al. 2009), BP-band formation is a simple marker for the evaluation of the anti-bone-resorptive effects of BPs in mice. The present data indicate that (i) as regards the anti-bone-resorptive effects of the non-N-BPs used in the present study, the relative potencies were oxidronate ≥ clodronate > etidronate ≈ medronate (Figs. 1 and 2A), and (ii) the anti-bone-resorptive effects of zoledronate + clodronate and zoledronate + oxidronate were much greater than those of zoledronate + etidronate and zoledronate + medronate. Medronate and oxidronate exert inflammatory and necrotic effects, unlike etidronate and clodronate. It should be noted that to attain the required anti-bone-resorptive effects, etidronate and clodronate are used at much higher doses than N-BPs (several hundred milligrams for etidronate and clodronate, but < 50 mg for N-BPs, see above). Although the incidence of ONJ is reported to be very low in patients given oral N-BPs such as alendronate, there is a report showing that even short-term use of oral alendronate can lead to ONJ in some patients (about 4%) after dental procedures such as extractions (Sedghizadeh et al. 2009). In particular, it is notable that the ratio of ONJ in patients receiving oral N-BPs to that in patients receiving intravenous N-BPs is higher in Japan than in the USA or EU (Urade 2010). Bone fractures occurring in patients with osteoporosis or ONJ are both serious matters, leading to a lowering of their quality of life. Thus, if possible, it is better to avoid or reduce both risks. For preventing the inflammatory and necrotic effects of N-BPs while retaining the desired and/or minimum anti-bone-resorptive effects, we propose that (i) clodronate may be useful as a combination drug with an oral N-BP, and (ii) etidronate may be useful as a substitution drug for N-BPs in patients who are thought to be at risk of ONJ (for example, patients under prolonged treatment with N-BPs, patients with periodontitis, and/or patients under consideration for tooth extraction). The case report we published on the effect of etidronate when used as a substitution drug for N-BPs seems to support the latter proposal (Yamaguchi et al. 2010).
Although direct evidence needs to be obtained in future studies, the pharmacological findings obtained in the present study, together with those obtained previously, suggest that (i) zoledronate may enter soft-tissue cells via the SLC20 and/or SLC34 phosphate transporters, (ii) oxidronate and pyrophosphate may enter via another family of phosphate transporters, possibly SLC17, and (iii) etidronate and clodronate may inhibit all of these transporters. Thus, our key conclusion is that at least in the present model, etidronate and clodronate protect against the inflammatory/necrotic effects of zoledronate, oxidronate, and pyrophosphate by inhibiting the various phosphate transporters that convey them into soft-tissue cells. Based on the effects of various drug-combinations on bone-resorption and on inflammatory/necrotic side effects, we propose that in certain patients (see above), clodronate and etidronate may be useful as a combination drug with an N-BP and as a substitution drug for N-BPs, respectively. The clinical aim in each case would be to limit or prevent inflammatory and necrotic effects while retaining the required and/or minimum anti-bone-resorptive effects.
This work was supported by grants from the Japan Society for the Promotion of Science (21390529, 20592318, and 21890019).
We are grateful to Dr. Robert Timms for editing the manuscript.
The authors declare no conflict of interest.