2013 Volume 231 Issue 3 Pages 159-164
2013 Volume 231 Issue 3 Pages 159-164
In extremely low birth weight (ELBW) infants, systemic hypotension is associated with poor neurological outcomes as a result of cerebral hypoperfusion. Treatment with arginine vasopressin (AVP) has been shown to increase blood pressure (BP) and urine output in ELBW infants suffering from refractory hypotension. The purpose of this study was to clarify whether low doses of AVP increased renal blood flow (RBF) in ELBW infants. We retrospectively analyzed data from the medical charts describing nine AVP infusions at 0.3-0.8 mU/kg/min in four ELBW infants. The median gestational age was 23 (22.5-23.5, interquartile range) weeks, and the median birth weight was 466 (414-563) g. Changes in the heart rate, BP, urine output, and RBF velocity patterns in response to the AVP infusions were compared using statistical analyses. The AVP infusion caused significant increases in systolic BP from 44 (41.0-47.0) to 50 (42.5-55.5) mmHg, diastolic BP from 17 (15.0-26.5) to 31 (28.5-33.0) mmHg, mean BP from 26 (24.5-30.5) to 36 (34.5-40.5) mmHg, and urine output from 1.4 (0.2-2.5) to 2.8 (1.0-8.6) mL/kg/hr. We also observed significant decreases in the resistance index from 1.0 (0.96-1.0) to 0.8 (0.71-0.91) and peak systolic flow velocity in the renal artery from 40 (27.2-50.6) to 28 (16.0-28.9) cm/s after AVP infusions. AVP infusions at 0.3-0.8 mU/kg/min in ELBW infants appeared to significantly increase the RBF by inducing renal vascular dilation and increasing the BP. Increasing the RBF most likely induces an increase in the glomerular filtration rate, resulting in the diuretic effect of AVP.
In extremely low birth weight (ELBW) infants, systemic hypotension is associated with poor neurological outcomes as a result of cerebral hypoperfusion (Volpe 2008). The causes of hypotension in ELBW infants are diverse, including septic shock, gastrointestinal perforation, intraventricular hemorrhage, and adrenal insufficiency. Recent studies have reported that arginine vasopressin (AVP) infusion is an effective treatment for ELBW patients exhibiting hypotension refractory to treatment with catecholamines and adrenocorticosteroids (Meyer et al. 2006; Bidegain et al. 2010; Ikegami et al. 2010). Treatment with AVP was originally shown to increase blood pressure (BP) in hypotensive adult patients with septic shock (Landry et al. 1997). In addition to increasing the BP, AVP at low doses (10 to 40 mU/min in adults) significantly increases the urine output (Holmes et al. 2001). Therefore, AVP infusion is a promising therapy for hypotension and oliguria, which are common problems in ELBW patients.
However, the mechanisms by which AVP infusion increases urine output have not been clarified. Although a few animal and in vitro studies have suggested that low doses of AVP induces renal artery vasodilation (Aki et al. 1994; Rudichenko and Beierwaltes 1995; Medina et al. 1996), no clinical studies have shown the renal vasodilatory effect of AVP.
We have clinically observed an increase in urine output and a decrease in the peak systolic velocity and resistance index (RI) in the renal artery in ELBW infants receiving AVP infusion therapy. The RI indicates the relative changes in vascular resistance. Therefore, we hypothesized that AVP infusion would increase the renal blood flow (RBF) by inducing renal vascular dilation, resulting in an increase in the glomerular filtration rate (GFR), and thereby causing the diuretic effect. The purpose of this study was to clarify whether AVP infusion at low doses (0.3-0.8 mU/kg/min) clinically dilated the renal artery and increased RBF in ELBW infants.
We retrospectively analyzed data from the medical charts of four ELBW infants admitted to the Tohoku University Hospital, Sendai, Japan, between June 2010 and September 2011. This study was approved by the ethics committee of the Tohoku University Graduate School of Medicine (No. 2012-1-77). Written informed consent was obtained from the parents of each infant. The inclusion criteria were as follows: (1) infants born with body weights of < 1,000 g, (2) inability to maintain vital organ blood flow despite treatment with catecholamines and adrenocorticosteroids, and (3) treatment with low doses of AVP.
The definition of the normal range of BP in very premature infants is controversial. It is, however, important to maintain vital organ flow, not to keep BP high. Therefore, vasopressor therapy was indicated in cases, in which vital organ blood flow, particularly to the brain, could not be adequately preserved. We initiated AVP when the RI in the anterior cerebral artery was higher than 0.75 or the diastolic flow velocity in the renal artery was zero, despite treatment with dopamine and adrenocorticosteroids. We did not use noradrenaline, which strongly increases cardiac muscle contractions, because the patients in this study had hypertrophic cardiac muscle due to twin-to-twin transfusion syndrome (TTTS) or treatment with adrenocorticosteroids and were assumed to have a low cardiac output because of hypertrophic cardiomyopathy. The dose of AVP infusion was at the discretion of the attending neonatologist, with reference to Ikegami et al who used AVP at a dose of 1-10 mU/kg/min (Ikegami et al. 2010). The patient heart rate, BP, urine volume, RI, and blood flow velocity pattern in the renal artery were statistically compared before and after AVP administration. The heart rate and BP just before AVP infusion were compared with those observed within 2 hours after AVP infusion. BP was measured using the IntelliVue MP50 Neonatal Monitor (Philips, Boeblingen, Germany) using oscillometry in all the cases except for one pair of measurements, which were performed using arterial monitoring with peripheral arterial catheters. BP was measured mostly using oscillometry because of the risk of interruption to peripheral circulation, and skin damage. The values of invasive versus non-invasive BP measurement have been reported to be in good agreement (Meyer et al. 2010). Urine output was determined by the increase in the weight of the infants’ diapers and was calculated at 2 hours before AVP infusion; this value was then compared with that obtained at 2 hours after AVP infusion. The blood flow velocity pattern and the RI in the renal and anterior cerebral artery were measured using pulsed-wave Doppler ultrasonography. The ultrasound examinations were performed using a 7-MHz Doppler transducer (Sonos 5500; Hewlett-Packard, Andover, MA). The measurements were obtained immediately before and within 5 hours after AVP administration and were performed by the attending neonatologists using a left renal artery lateral approach. Clear real-time ultrasound images of the artery were obtained at an angle of insonation of < 5° to the blood flow in the artery being studied. The RI was calculated using built-in software according to the following formula: RI = (peak systolic velocity − end diastolic velocity)/peak systolic velocity. Furthermore, we evaluated the incidence of a decreased serum sodium concentration, increased serum lactate levels and the development of necrotizing enterocolitis (NEC) as adverse effects of AVP.
DefinitionsChronic lung disease was defined as requiring additional oxygen at a gestational age of 36 weeks (Jobe and Bancalari 2001). TTTS was diagnosed using an ultrasound examination when cases of monochorionic diamniotic multiples defined at the first trimester showed both polyhydramnios of the maximum vertical pocket ≥ 8 cm in one sac and oligohydramnios of the maximum vertical pocket ≤ 2 cm in the other sac (Bruner et al. 1998). Sepsis was defined as systemic inflammatory response syndrome (SIRS) in response to an infectious process. SIRS was defined as the presence of two or more of the following: an abnormal body temperature, heart rate, respiratory rate, blood gas, or white blood cell count (Bone et al. 1992). Hemodynamically significant patent ductus arteriosus (PDA) was defined as echocardiographic evidence of a left-to-right ductal shunt and a dilated left atrium associated with the following clinical signs: tachycardia, wide pulse pressure, systemic hypotension, increase in oxygen and ventilator requirements, and radiographic evidence of cardiomegaly or pulmonary congestion (Su et al. 1997). NEC was defined as Bell stage 2 or greater, including any documentation of pneumatosis intestinalis, portal vein gas, or a surgically proven diagnosis (Bell et al. 1978).
Statistical analysisAll the values were expressed as the median (interquartile range). The Wilcoxon signed-rank test was used to compare the values for the clinical parameters before and after AVP infusions. Probability values of < 0.05 were considered to be statistically significant.
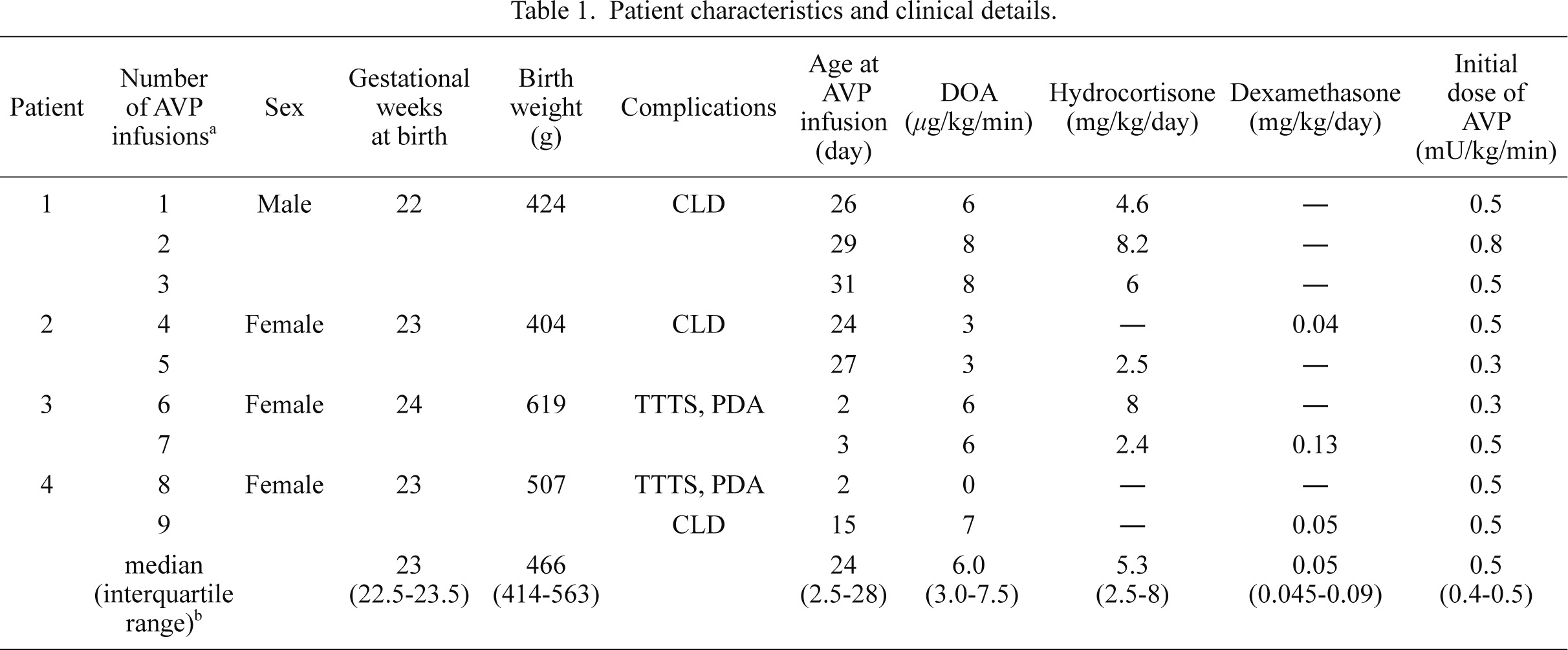
We identified nine separate vasopressin infusions in four ELBW infants (Table 1). All data (n = 9) retrieved from the medical charts of the included patients were available for review. The median gestational age was 23 (22.5-23.5) (median [interquartile range]) weeks, and the median birth weight was 466 (414-563) g; all the patients were extremely premature (born at < 25 gestational weeks). AVP infusion therapy was performed three times in patient 1 and twice in patients 2-4. The initial AVP infusion dose was 0.3-0.8 mU/kg/min. At the beginning of the AVP infusions, two patients had progressive chronic lung disease (patients 1 and 2), and two were TTTS recipients with PDA (patients 3 and 4). None of the patients had sepsis. The postnatal ages at the times of AVP administration were 24 to 31 days in patients 1 and 2, 2 and 3 days in patient 3, and 2 and 15 days in patient 4. Before each of the AVP infusions were initiated, we confirmed that the RI in the anterior cerebral artery was higher than 0.75 or the diastolic flow velocity in the renal artery was zero. Dopamine (3-8 μg/kg/min) and adrenocorticosteroid therapy (hydrocortisone, 2.4-8.2 mg/kg/day; or dexamethasone, 0.04-0.13 mg/kg/day) was used together with AVP in eight of the nine AVP infusions (Table 1).
Following the AVP infusions, no significant change was observed in the heart rate (152 [146-160] vs. 152 [147-158] bpm, P = 1.000). In contrast, significant increases were observed in systolic BP from 44 (41.0-47.0) to 50 (42.5-55.5) mmHg (P = 0.035), diastolic BP from 17 (15.0-26.5) to 31 (28.5-33.0) mmHg (P = 0.015), mean BP from 26 (24.5-30.5) to 36 (34.5-40.5) mmHg (P = 0.011) (Fig. 1), and urine output from 1.4 (0.2-2.5) to 2.8 (1.0-8.6) mL/kg/hr (P = 0.018) (Fig. 2). We also observed significant decreases in the RI values from 1.0 (0.96-1.0) to 0.8 (0.71-0.91) (P = 0.008) and the peak systolic flow velocity in the renal artery from 40 (27.2-50.6) to 28 (16.0-28.9) cm/s (P = 0.038) (Fig. 3) after the AVP infusions. No changes were observed in the serum sodium concentration (130 [126-137] vs. 133 [124-137] mEq/L, P = 0.594) or lactate levels (24.3 [19.9-33.9] vs. 26.9 [19.6-31.4] mg/dL, P = 0.374), and no patients developed NEC. However, among the patients shown in Table 1, patients 3 and 4 developed hemodynamically significant PDA as the BP increased, requiring PDA ligation on postnatal days 3 and 6, respectively. All the patients survived.
Patient characteristics and clinical details.
AVP, arginine vasopressin; DOA, dopamine; CLD, chronic lung disease; TTTS, recipient of twin to twin transfusion syndrome; PDA, patent ductus arteriosus.
aAVP infusion was performed a total of 9 times.
bvalues in the bottomline are expressed as the median (interquartile range).
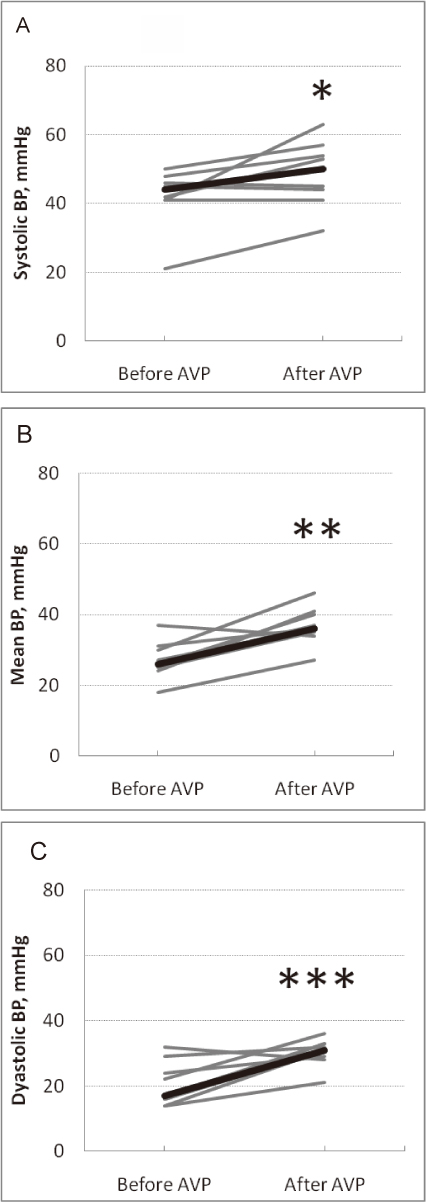
Changes in the blood pressure before and after AVP infusion.
Shown are the changes in the systolic (A), mean (B) and diastolic (C) blood pressure before and after AVP infusion (gray bars, individuals [n = 9]; bold black bar, median).
*P = 0.035, **P = 0.011, and ***P = 0.015 [Wilcoxon signed-rank test] compared with the values before and after AVP infusion.

Changes in urine output before and after AVP infusion.
Shown are the changes in urine output before and after AVP infusion (gray bars, individuals [n = 9]; bold black bar, median).
*P = 0.018 [Wilcoxon signed-rank test] compared with the values before and after AVP infusion.

Changes in RI and peak systolic velocity in the renal artery before and after AVP infusion.
Shown are the changes in RI (A) and peak systolic velocity (B) in the renal artery before and after AVP infusion (gray bars, individuals [n = 9]; bold black bar, median).
*P = 0.008, **P = 0.038 [Wilcoxon signed-rank test] compared with the values before and after AVP infusion.
Following the AVP infusions in ELBW infants, a significant increase in the BP and urine output and a significant decrease in the RI and peak systolic flow velocity in the renal artery were observed. These results suggested, first, that AVP infusion increased the RBF because the mean flow in the blood vessels is generally expressed as F = P/R (where F = flow, P = pressure, and R = resistance). Second, the increase in the RBF was probably the results of renal vasodilation because the mean flow in the blood vessels is generally expressed as A = F/V (where F = flow, A = area of conduct, and V = velocity). However, in this study, because no significant change was observed in the mean velocity, the contribution of the decrease in the velocity might have been relatively small. Meanwhile, we also speculated on the occurrence of renal vasodilation using another formula: R = 8ηL/πr4 (where R = resistance, η = viscosity and L = length, r = radius). When the RI decreased, the radius was thought to have increased because the length of the vessels and the viscosity (hematocrit) did not change when measurements obtained before and after AVP infusions were compared (Barrett et al. 2010). Therefore, the RBF must have increased as a result of renal vascular dilation. We estimated the RBF using the above formula because precise measurements of the RBF in ELBW infants are too difficult to perform. Additionally, in this study, as dopamine (3-8 μg/kg/min) was used together with AVP in eight of the nine AVP infusions, we must consider the renal vasodilatory effects of dopamine. However, an increase in the BP and urine output and a decrease in the RI were not observed when measurements obtained before and after dopamine infusions were compared. Therefore, we speculated that the increase in the RBF and the renal vasodilation were not the effect of dopamine, but of AVP.
AVP reportedly has an antidiuretic effect on the kidneys. In contrast, AVP at low doses (0.01 to 0.04 U/min) reportedly increases the urine output while increasing the BP in adult patients with advanced vasodilatory shock (Holmes et al. 2001). However, the mechanisms of the diuretic effect of AVP have not been fully clarified in clinical practice. In this study, we clinically confirmed for the first time that low doses of AVP increased RBF through renal vascular dilation, whereas the mechanisms by which the renal artery was dilated were unclear. This finding is consistent with the results of previous animal and in vitro studies, in which low doses of AVP induced renal vasodilation and increased RBF. Rudichenko and Beierwaltes (1995) reported that AVP induced renal vasodilation through nitric oxide (NO) synthesis in the rat kidney because vasodilation was eliminated by prior NO synthesis inhibition with L-nitromonomethylarginine (L-NAME). Aki et al. (1994) reported that AVP induced NO mediated renal vasodilation in dogs through V2 receptors after pretreatment with a V1 antagonist, inhibiting vasoconstrictor activity. Additionally, Medina et al. (1996) reported that AVP caused the prostaglandin-mediated dilation of human renal arteries following a nephrectomy only in the presence of V1 receptor inhibition. Therefore, we speculated that low doses of AVP could increase the RBF through renal vascular dilation mediated by NO, thereby increasing the GFR and urine output in ELBW infants.
In recent studies, transient adrenocortical insufficiency secondary to an immature hypothalamic-pituitary-adrenal axis has been frequently reported in preterm infants with hypotension, and adrenocorticosteroid replacement has been required in these infants (Watterberg et al. 2001; Ng et al. 2006). In this study, we did not examine the blood or urine levels of cortisol or AVP metabolites because we could not obtain sufficient samples from the very small and extremely premature infants. However, the four cases in the present study were premature and sickly, similar to the reported patients with adrenocortical insufficiency. We speculated that the patients in this study might have also had a background of adrenocortical insufficiency. Furthermore, the increased BP after AVP infusion was unlikely to be due to delayed steroid effects because the half-lives of hydrocortisone and dexamethasone are about 1.5 and 5.6 hours, respectively, and more than three days had elapsed between the administration of steroids and that of AVP except for one AVP administration, which was performed two hours after the administration of dexamethasone. Therefore, the patients in this study might have developed both cortisol and AVP deficiencies, and AVP infusion may physiologically serve as a replacement therapy for extremely premature ELBW infants who are born at the limits of viability.
This study was limited by its retrospective nature and small sample size. The number of ELBW infants who develop hypotension refractory to treatment with catecholamines and adrenocorticosteroids is very small, and we were only able to describe 9 AVP infusions in four ELBW infants in this study. As a first step in clarifying the diuretic mechanisms, we propose that low doses of AVP might increase the RBF by inducing renal vascular dilation and increasing the BP by constricting constriction of peripheral vessels. Moreover, AVP administration did not decrease the serum sodium concentration, increase the lactate levels, or induce NEC, all of which have been reported as acute adverse effects of AVP infusion. However, to evaluate mesenteric ischemia closely, the blood flow patterns of the celiac artery should be examined. Some studies suggest that AVP causes mesenteric ischemia in a dose-dependent manner (Holmes et al. 2001). Establishing the minimum dose capable of producing therapeutic effects is important. Additionally, in patients 3 and 4, the PDA became hemodynamically significant with the increase in BP and required ligation. Although AVP has not been reported to induce patency of the the ductus arteriosus, the increase in the systemic BP might influence the increase in the L-R shunt.
Further experience and research evaluating the use of AVP infusion are required to determine the mechanisms underlying its effects, as well as appropriate doses and indications. Additionally, multi-center prospective studies are warranted.
In conclusion, AVP infusions at 0.3-0.8 mU/kg/min in ELBW infants significantly increased the RBF by inducing renal vascular dilation and increasing the BP by constricting the peripheral vessels. Increasing the RBF most likely increases the GFR, resulting in the diuretic effect of AVP. The results of this study indicate that AVP infusion could be a useful strategy for the management of hypotension in extremely premature ELBW infants.
The authors declare no conflict of interest.