Abstract
Ghrelin is a novel growth hormone-releasing peptide isolated from the stomach and possesses various cardioprotective effects, including energy balance improvement and regulation of autonomic nervous system activity. We investigated the changes in serum ghrelin levels and its association with cardiac function and myocardial infarct size in patients with acute myocardial infarction (AMI). Forty-seven consecutive patients were divided into the following 4 groups: 16 patients with AMI, 12 patients with unstable angina pectoris (UAP), 13 patients with stable angina pectoris (SAP), and 6 control patients. Serum levels were measured with the ELISA kit. Compared to the control (72 ± 26 fmol/mL), SAP (69 ± 47 fmol/mL), and UAP (72 ± 31 fmol/mL) groups, serum ghrelin levels on admission were significantly lower in the AMI group (27 ± 12 fmol/mL, P < 0.01). After admission, the serum ghrelin level gradually increased (30 ± 15 fmol/mL on day 2 and 39 ± 18 fmol/mL on day 7) and became significantly higher on day 14 (49 ± 28 fmol/mL, P < 0.01), compared to the level on admission. In patients with AMI, the ratio of day 14 to admission serum ghrelin levels, an index of AMI-related acute changes in ghrelin, correlated positively with peak creatine phosphokinase levels (R = 0.72, P < 0.01) and the double products (R = 0.60, P < 0.01) and inversely with left ventricular ejection fraction (R = −0.53, P < 0.05). In conclusion, serum ghrelin levels are significantly decreased in association with myocardial infarct size and cardiac function.
Introduction
In patients with acute myocardial infarction (AMI), one of the presenting symptoms is loss of appetite (Patel et al. 2004). Increased sympathetic activity in AMI has also been documented and may contribute to the progression of myocardial injury and the development of lethal ventricular tachyarrhythmia (Schömig 1990).
Ghrelin is a novel growth hormone (GH)-releasing peptide, originally isolated from the stomach, which has been identified as an endogenous ligand for the GH secretagogue receptor (GHS-R) (Kojima et al. 1999). GHS-R mRNA is found not only in the hypothalamus and pituitary gland but also in the heart and blood vessels (Gnanapavan et al. 2002). Significant evidence for a cardiovascular function of ghrelin and its role in the regulation of autonomic activity has been reported (Soeki et al. 2008; Kishimoto et al. 2012; Mao et al. 2012). Although recent experimental studies have demonstrated that administration of ghrelin could protect against ischemia and reperfusion injury, attenuate post-infarction ventricular dysfunction and remodeling, and improve the prognosis of myocardial infarction and heart failure (Nagaya et al. 2001; Chang et al. 2004; Schwenke et al. 2008; Soeki et al. 2008; Mao et al. 2012), the role of ghrelin in the clinical setting remains unknown.
The present study was therefore designed to investigate the changes in serum ghrelin levels and its association with cardiac function and myocardial infarct size in AMI patients.
Methods
Study Subjects
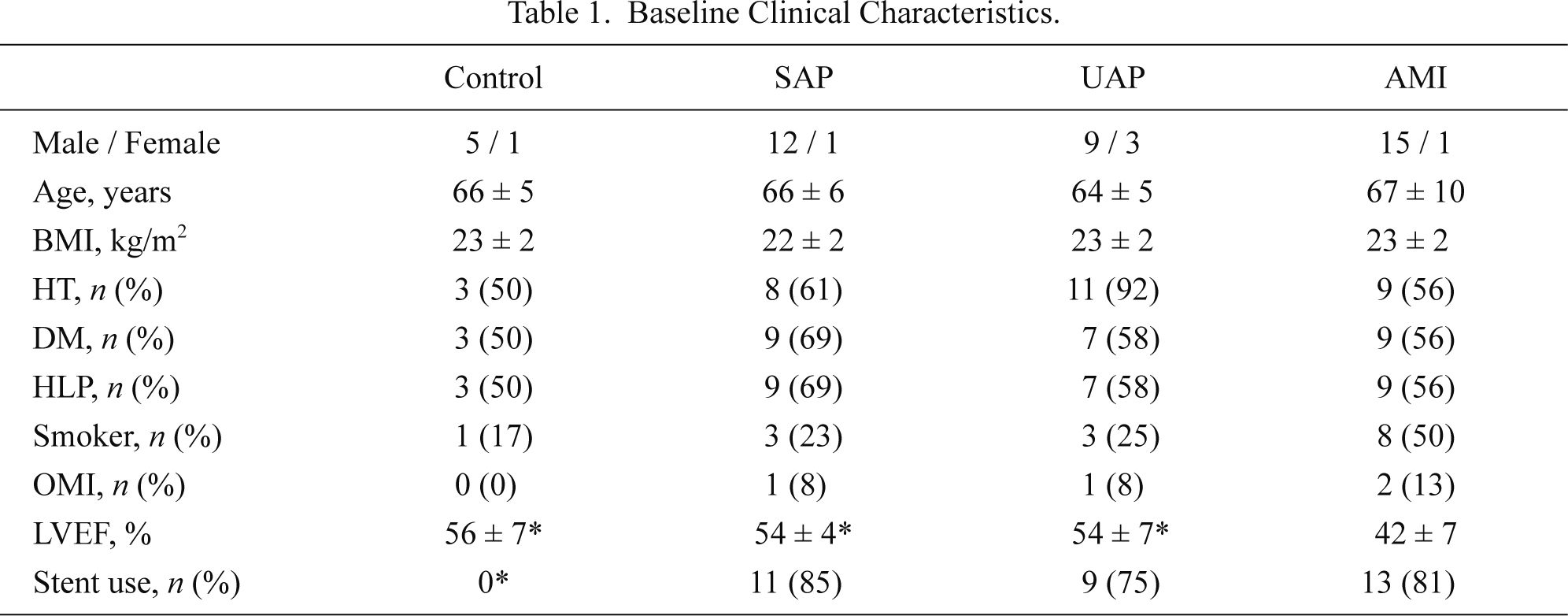
We investigated 89 patients with chest pain who suspected of coronary artery disease and admitted to the Coronary Care Unit in the National Cerebral and Cardiovascular Center (Suita, Osaka, Japan) between March and May 2003 in a prospective manner. Approval to conduct the study was obtained from the ethics committee of the National Cerebral and Cardiovascular Center. After 42 patients with left main trunk stenosis, cardiogenic shock, and severe kidney dysfunction (serum creatinine ≥ 1.5 mg/dL) and without informed consent were excluded, a total of 47 patients were included in the present study and divided into the following four groups; AMI (n = 16), unstable angina pectoris (UAP, n = 12), stable angina pectoris (SAP, n = 13) and control without significant ( ≥ 50% stenosis) coronary artery stenosis (n = 6). All patients with AMI, UAP or SAP were successfully treated with percutaneous coronary intervention (PCI). The diagnosis of AMI was based on the following criteria; chest pain for more than 30 min; at least 0.1 mV of ST segment elevation in two adjacent electrocardiogram leads; admission to hospital within 24 hours of the onset of symptoms. SAP was defined as the presence of coronary artery disease with stable chest symptoms. UAP was defined as the presence of new-onset angina, angina at rest or angina of increasing frequency without the elevation of cardiac enzyme. All patients had no evidence of active infection, gastric ulcer, or other primary cachectic state such as cancer or thyroid disease, and severe liver disease. From all patients written informed consent was obtained with measurements of body mass index (calculated as body weight in kilograms divided by the square of the height in meters), blood pressure and heart rate.
Blood Sampling and Assay for Serum Ghrelin
In the control and SAP groups, blood samples were taken from the antecubital vein on the morning after an overnight fast. In the UAP and AMI groups, blood samples were taken on admission and before treatment. In the AMI group, blood samples were also taken on day 2, day 7, and day 14. Serum samples were prepared as previously described (Akamizu et al. 2005). Serum levels of unacylated ghrelin (desacyl ghrelin) were measured with the commercially available desacyl-ghrelin ELISA kit according to the manufacturer’s protocol (Mitsubishi Kagaku, Tokyo, Japan). The lower limit of detection for desacyl ghrelin in this assay system is 12.5 fmol/mL. The intra- and inter-assay coefficients of variation are 3.7% and 8.1% for desacyl ghrelin, respectively (Akamizu et al. 2006).
Cardiac Catheterization, Laboratory and Echocardiographic Measurements
As reported previously (Kataoka et al. 2010), selective coronary angiography was performed in multiple projections after intracoronary administration of nitroglycerin (0.125-0.25 mg). In patients treated with PCI, all procedural decisions, including device selection and intravascular ultrasound, were made at the discretion of the individual PCI operator. Intravenous heparin (5,000 IU) and intracoronary nitroglycerin (0.5 mg) were administered before PCI. Procedural success was defined as residual stenosis < 50% without major complications (i.e., AMI, need for bypass surgery or repeat PCI, or death). Following PCI, left ventriculography was performed to measure end-diastolic and end-systolic volumes and ejection fraction (LVEF) using the area-length method (QLV-CMS version 5.0, MEDIS Medical Imaging Systems, Leiden, the Netherlands) (Sheehan et al. 1986). In addition, creatine phosphokinase (CPK) and CPK-MB levels were measured on admission and at 3-hour intervals until CPK and CPK-MB levels peaked. Glycemic and lipid profile, and c-reactive protein (CRP) levels were also measured. Echocardiographic analysis was performed to determine left ventricular dimension and fractional shortening at admission and follow-up period.
Statistical Analysis
Continuous variables are expressed as means ± s.d. Comparisons of clinical parameters among the 4 groups were performed by one-way analysis of variance. Correlation coefficients between serum ghrelin levels and clinical variables were calculated by linear regression analysis. P < 0.05 was considered to indicate statistical significance.
Results
Baseline Characteristics
There were no significant differences in age, sex, body mass index, and the prevalence of coronary risk factors among the 4 groups (Table 1). Also, heart rate, blood pressure and laboratory data except CRP were comparable among the 4 groups (Table 2). LVEF was lower and CRP levels were higher in the AMI group than other 3 groups (Tables 1 and 2), whereas the number of diseased vessels and the percentage of PCI with stent use were comparable among the SAP, UAP, and AMI groups. In the AMI group, elapsed time from the onset of AMI was 7 ± 5 hours and peak CPK levels were 3,088 ± 3,489 U/L. The infarct site was anterior in 9 patients, inferior in 4 patients, and posterior in 5 patients. Medical treatment following AMI includes anti-platelet agents (n = 16), angiotensin-converting enzyme inhibitors (n = 11), β-blockers (n = 2), statins (n = 5) and anti-diabetic agents (n = 4).
Serial Changes in Serum Ghrelin Levels and Left Ventricular Function
The serum ghrelin level on admission was significantly lower in the AMI group (27 ± 12 fmol/ml) (Fig. 1), whereas it was comparable among the non-AMI groups, i.e., the control (72 ± 26 fmol/ml), SAP (69 ± 47 fmol/ml), and UAP (72 ± 31 fmol/ml) groups. The serum ghrelin levels were rather not associated with risk factors including high density lipoprotein cholesterol, low density lipoprotein cholesterol, total cholesterol, triglyceride and glucose levels in the overall patients.
Fig. 2 plots the time of the first and second blood sampling and serum ghrelin levels in patients with AMI. Fig. 3 shows the serial changes in serum ghrelin levels in the AMI group. The serum ghrelin level gradually increased (30 ± 15 fmol/mL on day 2 and 39 ± 18 fmol/mL on day 7) and became significantly higher on day 14 (49 ± 28 fmol/mL, P < 0.01), compared to the level on admission. The serum ghrelin level gradually increased and became significantly higher on day 14, compared to the level on admission. In addition, the ratio of serum ghrelin levels on day 7 and day 14 to that on admission was significantly increased in comparison with that on admission (Fig. 4).
In patients with AMI, serum ghrelin levels on day 14 divided by that on admission correlated positively with peak creatinine phosphokinase levels (R = 0.72, P < 0.01) and inversely with LVEF (R = −0.53, P < 0.05) (Fig. 5A and B). This index of AMI-related acute changes in ghrelin levels also correlated positively with the double products (systolic blood pressure multiplied by heart rate on admission; mmHg/min) (R = 0.60, P < 0.05), but not with body mass index (Fig. 6A and B) and CRP levels (at baseline and maximum levels, and those differences).
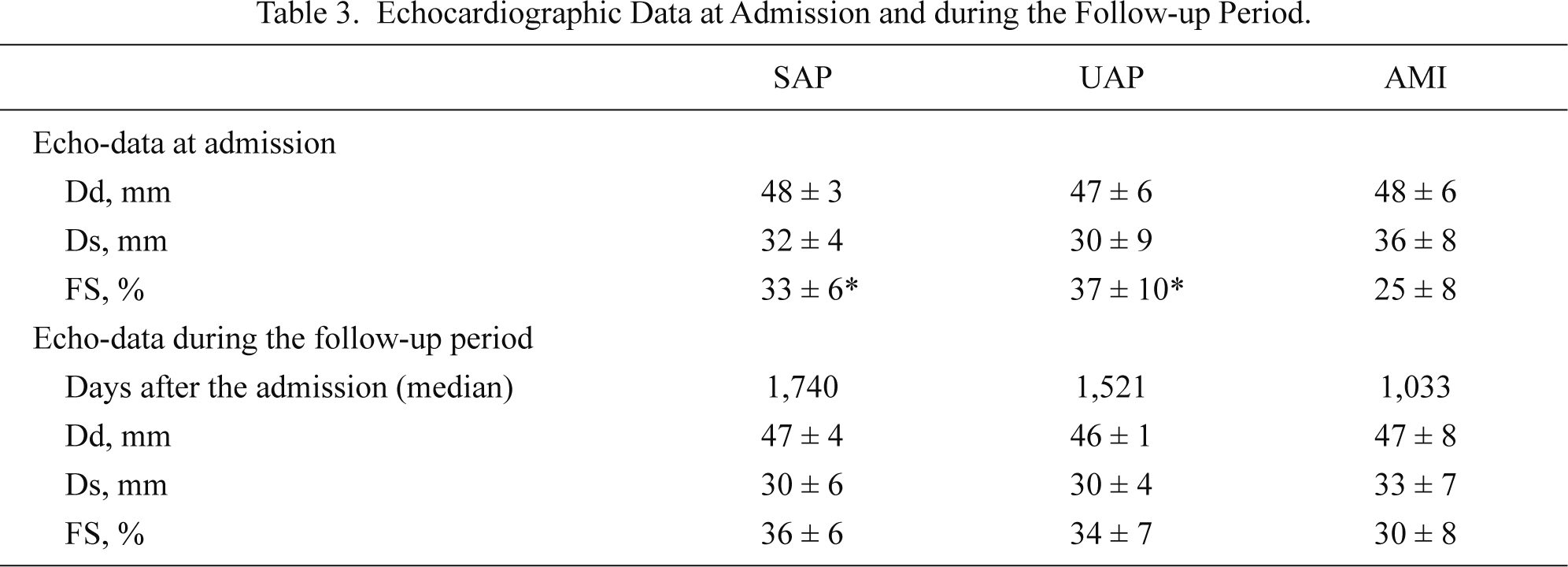
Table 3 shows left ventricular function evaluated by echocardiography at admission and the follow-up period in SAP, UAP and AMI groups. Fractional shortening was significantly (P < 0.05) lower at admission and remained tended to be reduced (P = 0.08) at the follow-up in AMI group than SAP and UAP groups. The index of AMI-related acute changes in ghrelin did not correlated with changes in left ventricular dimension between the baseline and the follow-up.
Discussion
The major findings of the present study using a sensitive ELISA kit are: (1) serum ghrelin levels are significantly lower on admission in AMI patients than control patients with no coronary artery disease and patients with SAP or UAP; (2) ghrelin levels gradually recovered during the 2 weeks following AMI; and (3) the ratio of day 14 to admission serum ghrelin levels, an index of AMI-related acute changes in ghrelin levels, correlated positively with peak CPK levels and the double product, and inversely with LVEF.
Decreased Serum Ghrelin Levels in Patients with AMI
Ghrelin, a 28-amino acid peptide, was isolated from the human and rat stomach and identified as an endogenous ligand for the GHS-R (Kojima et al. 1999). The ghrelin system is ubiquitously expressed and participates in the regulation of appetite, energy, bodyweight, metabolism of glucose and fat, as well as modulation of gastrointestinal function and cell proliferation or apoptosis (Zhang et al. 2010; Kishimoto et al. 2012). In addition, mounting experimental evidence suggests that ghrelin has a close relationship with the cardiovascular system (Zhang et al. 2010; Kishimoto et al. 2012).
In the previous study with only one-point, desacyl ghrelin non-specific measurement, serum ghrelin levels decreased in AMI patients in comparison with the healthy control, although there were no data of time interval between the onset of AMI and blood sampling (Kadoglou et al. 2010). Therefore, this is the first clinical study investigating serial changes in serum ghrelin levels in patients following AMI. Interestingly, serum ghrelin levels were significantly decreased at the onset of myocardial infarction and recovered gradually over weeks to levels comparable with non-AMI patients with stable or unstable angina or no coronary artery disease.
Association of Changes in Ghrelin Levels with Myocardial Infarct Size and Cardiac Function
The present study also demonstrated that the ratio of day 14 to admission serum ghrelin levels, an index of AMI-related acute changes in ghrelin, correlated positively with peak CPK levels and the double product, and inversely with LVEF. Although these findings do not address a cause-and-effect relationship, several possibilities could be discussed. Friberg and colleagues (2000) reported that GH secretion was stimulated in patients with AMI, especially in patients with severe cardiac damage (Friberg et al. 2000). Since ghrelin is a GH-releasing peptide, increased GH levels may decrease ghrelin secretion due to the negative feedback loop of GH.
Decreased ghrelin levels may be related in part to extensive myocardial damage in AMI patients (Fig. 5A and B). It is reported that administration of ghrelin protects the heart against ischemia and reperfusion injury via ERK1/2 and PI3K/Akt-dependent pathways (Chang et al. 2004). Also, in an animal model of AMI, ghrelin effectively inhibits cardiac sympathetic activity, prevents ventricular tachyarrhythmias, and reduces mortality (Schwenke et al. 2008; Soeki et al. 2008). In the present study, ghrelin levels were well preserved in patients with lower double products (Fig. 6A).
Considering these cardioprotective effects of ghrelin, its exogenous administration may represent a new approach for the treatment of AMI with severely depressed left ventricular function. Indeed, in patients with heart failure, Nagaya et al. (2004) demonstrated that exogenous ghrelin administration induces beneficial hemodynamic effects via reducing cardiac afterload and increasing cardiac output without an increase in heart rate.
Limitations
The interpretation of our results should take into consideration several limitations. First, the number of study patients is small. Second, the mechanism by which AMI suppresses ghrelin secretion remains unknown because this was not a mechanistic study. Therefore, larger study is needed for the confirmation of our results in the future. Third, in all of the UAP and AMI groups, the first blood samples were not taken after the overnight fast. Possibility remains whether difference from the control and SAP groups with fasted blood samples may affect the result. However, Fig. 2 shows that serum ghrelin levels were low and comparable between the first (obtained at admission) and the second sampling (obtained in fasting condition) except one patient.
Conclusion
The present study demonstrates that serum ghrelin levels are significantly decreased in association with myocardial infarct size and cardiac function in patients with AMI. Taking the various cardioprotective actions of ghrelin into account, the present findings support further study of ghrelin administration as a therapeutic modality for AMI patients.
Acknowledgements
We thank Mrs. Hiromi Maeda and Dr. Nobuhito Yagi for their assistance. The present work was supported by a Grant-in-Aid for Scientific Research on Innovative Areas (Research in a proposed research area) “Molecular Basis and Disorders of Control of Appetite and Fat Accumulation,” Grant-in-Aid for Scientific Research (18890018), the global COE project (F02), and Grants-in-Aid (H22-Shinkin-004) from the Japanese Ministry of Education, Culture, Sports, Science and Technology in Tokyo, Japan.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Akamizu,
T.,
Murayama,
T.,
Teramukai,
S.,
Miura,
K.,
Bando,
I.,
Irako,
T.,
Iwakura,
H.,
Ariyasu,
H.,
Hosoda,
H.,
Tada,
H.,
Matsuyama,
A.,
Kojima,
S.,
Wada,
T.,
Wakatsuki,
Y.,
Matsubayashi,
K., et al.
(2006) Plasma ghrelin levels in healthy elderly volunteers: the levels of acylated ghrelin in elderly females correlate positively with serum IGF-I levels and bowel movement frequency and negatively with systolic blood pressure. J. Endocrinol., 188, 333-344.
-
Akamizu,
T.,
Shinomiya,
T.,
Irako,
T.,
Fukunaga,
M.,
Nakai,
Y.,
Nakai,
Y. &
Kangawa,
K.
(2005) Separate measurement of plasma levels of acylated and desacyl ghrelin in healthy subjects using a new direct ELISA assay. J. Clin. Endocrinol. Metab., 90, 6-9.
-
Chang,
L.,
Ren,
Y.,
Liu,
X.,
Li,
W.G.,
Yang,
J.,
Geng,
B.,
Weintraub,
N.L. &
Tang,
C.
(2004) Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J. Cardiovasc. Pharmacol., 43, 165-170.
-
Friberg,
L.,
Werner,
S.,
Eggertsen,
G. &
Ahnve,
S.
(2000) Growth hormone and insulin-like growth factor-1 in acute myocardial infarction. Eur. Heart J., 21, 1547-1554.
-
Gnanapavan,
S.,
Kola,
B.,
Bustin,
S.A.,
Morris,
D.G.,
McGee,
P.,
Fairclough,
P.,
Bhattacharya,
S.,
Carpenter,
R.,
Grossman,
A.B. &
Korbonits,
M.
(2002) The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab., 87, 2988-2991.
-
Kadoglou,
N.P.E.,
Lampropoulos,
S.,
Kapelouzou,
A.,
Gkontopoulos,
A.,
Theofilogiannakos,
E.K.,
Fotiadis,
G. &
Kottas,
G.
(2010) Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease: KOZANI STUDY. Transl. Res., 155, 238-246.
-
Kataoka,
Y.,
Miyazaki,
S.,
Yasuda,
S.,
Nagaya,
N.,
Noguchi,
T.,
Yamada,
N.,
Morii,
I.,
Kawamura,
A.,
Doi,
K.,
Miyatake,
K.,
Tomoike,
H. &
Kangawa,
K.
(2010) The first clinical pilot study of intravenous adrenomedullin administration in patients with acute myocardial infarction. J. Cardiovasc. Pharmacol., 56, 413-419.
-
Kishimoto,
I.,
Tokudome,
T.,
Hosoda,
H.,
Miyazato,
M. &
Kangawa,
K.
(2012) Ghrelin and cardiovascular diseases. J. Cardiol., 59, 8-13.
-
Kojima,
M.,
Hosoda,
H.,
Date,
Y.,
Nakazato,
M.,
Matsuo,
H. &
Kangawa,
K.
(1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature, 402, 656-660.
-
Mao,
Y.,
Tokudome,
T.,
Otani,
K.,
Kishimoto,
I.,
Nakanishi,
M.,
Hosoda,
H.,
Miyazato,
M. &
Kangawa,
K.
(2012) Ghrelin prevents incidence of malignant arrhythmia after acute myocardial infarction through vagal afferent nerves. Endocrinology, 153, 3426-3434.
-
Nagaya,
N.,
Moriya,
J.,
Yasumura,
Y.,
Uematsu,
M.,
Ono,
F.,
Shimizu,
W.,
Ueno,
K.,
Kitakaze,
M.,
Miyatake,
K. &
Kangawa,
K.
(2004) Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation, 110, 3674-3679.
-
Nagaya,
N.,
Uematsu,
M.,
Kojima,
M.,
Ikeda,
Y.,
Yoshihara,
F.,
Shimizu,
W.,
Hosoda,
H.,
Hirota,
Y.,
Ishida,
H.,
Mori,
H. &
Kangawa,
K.
(2001) Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation, 104, 1430-1435.
-
Patel,
H.,
Rosengren,
A. &
Ekman,
I.
(2004) Symptoms in acute coronary syndromes: does sex make a difference? Am. Heart J., 148, 27-33.
-
Schömig,
A.
(1990) Catecholamines in myocardial ischemia. Systemic and cardiac release. Circulation, 82 (3 Suppl), 13-22.
-
Schwenke,
D.O.,
Tokudome,
T.,
Kishimoto,
I.,
Horio,
T.,
Shirai,
M.,
Cragg,
P.A. &
Kangawa,
K.
(2008) Early ghrelin treatment after myocardial infarction prevents an increase in cardiac sympathetic tone and reduces mortality. Endocrinology, 149, 5172-5176.
-
Sheehan,
F.H.,
Bolson,
E.L.,
Dodge,
H.T.,
Mathey,
D.G.,
Schofer,
J. &
Woo,
H.W.
(1986) Advantages and applications of the centerline method for characterizing regional ventricular function. Circulation, 74, 293-305.
-
Soeki,
T.,
Kishimoto,
I.,
Schwenke,
D.O.,
Tokudome,
T.,
Horio,
T.,
Yoshida,
M.,
Hosoda,
H. &
Kangawa,
K.
(2008) Ghrelin suppresses cardiac sympathetic activity and prevents early left ventricular remodeling in rats with myocardial infarction. Am. J. Physiol. Heart Circ. Physiol., 294, H426-432.
-
Zhang,
G.,
Yin,
X.,
Qi,
Y.,
Pendyala,
L.,
Chen,
J.,
Hou,
D. &
Tang,
C.
(2010) Ghrelin and cardiovascular diseases. Curr. Cardiol. Rev., 6, 62-70.