2013 Volume 231 Issue 4 Pages 265-270
2013 Volume 231 Issue 4 Pages 265-270
Renal biopsy is the gold standard for confirmation of disease severity and diagnosis of glomerulonephritis (GN), but its procedure is invasive with a risk of complications. Thus, a non-invasive monitoring method is desirable especially in pediatric patients. Fractalkine and monocyte chemoattractant protein-1 (MCP-1) are proinflammatory chemokines, and both have been reported to be involved in the pathogenesis of immunocomplex-mediated GN. Recently, it has been reported that urinary fractalkine and MCP-1 may serve as possible predictors of disease activity of adult patients with GN. We, therefore, examined whether urinary levels of fractalkine and MCP-1 correlate with clinical and histologic parameters. Twenty-six consecutive children with various types of GN were enrolled in this study, including lupus nephritis, IgA nephropathy, membranous nephropathy, acute GN, and thin basement membrane disease (served as a non-inflammatory control). Urinary fractalkine and MCP-1 concentrations in the first morning urine samples obtained at the time of renal biopsy were measured by enzyme-linked immunosorbent assay, and standardized for urinary creatinine concentrations. Urinary fractalkine concentration differed significantly among the disease categories. Urinary concentrations of fractalkine and MCP-1 showed a significant positive correlation with the degree of occult blood in urine and a significant inverse correlation with the estimated glomerular filtration rate. Furthermore, the urinary MCP-1 concentration was significantly correlated with histological chronicity indices in patients with lupus nephritis and IgA nephropathy. Measurement of urinary fractalkine and MCP-1 concentrations may be useful as a non-invasive method for predicting the disease activity of GN in children.
Renal biopsy is the gold standard for confirmation of disease severity and activity as well as diagnosis of glomerulonephritis (GN), but its procedure is invasive with a risk of potential complications. Thus, a non-invasive monitoring method is desirable in patients with kidney disease, especially pediatric-onset GN. Recently, it has been reported that the gene expression of functional molecules, such as transcriptional factors and proinflammatory cytokines/chemokines, in urinary sediment reflects pathogenesis and disease activity in patients with lupus nephritis (LN), IgA nephropathy (IgAN) and purpura nephritis (PN), and could be used for non-invasive monitoring of disease activity and response to treatment, without the potential demerits of invasive renal biopsy (Chan et al. 2003; Wang and Szeto 2007; Tsuruga et al. 2010, 2011). However, measurement of messenger RNA in urinary sediment is not always possible for routine examination because of RNA instability (Szeto et al. 2012). Alternatively, recent studies have reported the usefulness of determining the “stable” urinary protein levels of functional molecules in patients with glomerular diseases (Stangou et al. 2009, 2013; Watson et al. 2012; Abujam et al. 2013; Korzeniecka-Kozerska et al. 2013). Among such candidate urinary cytokines/chemokines, monocyte chemoattractant protein-1 (MCP-1) has been reported to be a possible biomarker reflecting glomerular inflammation in patients with LN and IgAN (Stangou et al. 2009, 2013; Watson et al. 2012; Abujam et al. 2013), since glomerular expression of MCP-1 is actually involved in the pathogenesis and subsequent tissue injury (Marks et al. 2008).
On the other hand, we recently confirmed that fractalkine/CX3CL1, a chemokine acting as a chemoattractant for active monocytes/neutrophils expressing its receptor, is involved in the pathogenesis and aggravation of childhood IgAN, PN and LN (Aizawa-Yashiro et al. 2013; Tanaka and Imaizumi 2013). Interestingly, it has been reported that measurement of urinary fractalkine levels is useful for monitoring of kidney transplant patients, since only changes in urinary fractalkine differentiate patients with acute rejection from those with acute tubular necrosis (Peng et al. 2008). Furthermore, it has recently been reported that upregulation of glomerular and urinary expression of fractalkine promotes the onset of gross hematuria in adult patients with IgAN (Cox et al. 2012). However, the implication of urinary fractalkine concentration in children with GN remains to be determined. Accordingly, the present study was conducted to explore whether the urinary concentrations of fractalkine and MCP-1 correlate with clinical and histological parameters in children with several forms of GN, since non-invasive monitoring method is desirable in pediatric patients with kidney disease.
Twenty-six consecutive children with various types of GN were enrolled in this study, including LN (n = 6), IgAN (n = 14), membranous nephropathy (MN, n = 2), acute GN (AGN, n = 2), and thin basement membrane disease (TBMD, n = 2), a non-inflammatory kidney disease. The present study was approved by the ethics committee of Hirosaki University Graduate School of Medicine, Hirosaki, Japan (2012-177).
A whole-stream early morning urine specimen at the time of renal biopsy was collected from each subject for enzyme immunosorbent assay (ELISA) study. Samples were centrifuged at 3,000 × g for 30 minutes, and aliquots of the urine supernatant were stored at −80°C until analysis. Concentrations of the soluble form of Fkn and MCP-1 in urine were quantified using pre-coated ELISA kits (R & D Systems, Minneapolis, MN). These assays have been commercially validated for use in urine. The data for the concentrations of both urinary chemokines were standardized against urinary creatinine concentration, and expressed as units per gram creatinine (gCre).
On the day of urine collection, the urinary protein/urinary creatinine ratio (Up/Ucr), grade of occult blood in urine (OB) on a scale of 0-3 [0, none; 1, about 20 red blood cells (RBCs)/μl; 2, about 80 RBCs/μl; and 3, about 200 RBCs/μl], and estimated glomerular filtration rate (eGFR) determined using Schwartz’s formula (Schwartz 1992) were assessed for comparison with the concentrations of urinary fractalkine and MCP-1. The biopsy specimens obtained from patients with immunocomplex-mediated mesangial proliferative form of GN (defined as MesPGN, such as proliferative LN and IgAN in this study, n = 18) were scored semiquantitatively in a blinded manner by one of the authors, using the scoring system for childhood IgAN described by Andreoli and Bergstein (1989). The activity index (AI) was determined by grading mesangial proliferation, interstitial mononuclear cell infiltration, and cellular crescent formation using a 4-grade scale (0, none; 1, mild; 2, moderate; 3, severe), on the basis of the percentage of glomeruli involved (0, 0%; 1, 1-20%; 2, 21-50%; 3, > 50%). The chronicity index (CI) was determined by counting the number of glomeruli demonstrating fibrous crescents and segmental or global sclerosis, each being scored on a scale of 0-3, on the basis of the percentage of glomeruli involved as indicated. Tubular atrophy and interstitial fibrosis were each scored on a scale of 0-3. The sum of these numbers comprised the AI (maximum = 9) and the CI (maximum = 12), respectively.
Statistical analysis was performed using SPSS for Windows (SPSS, Chicago, IL), and the results were presented as mean ± s.d. unless otherwise specified. The urinary chemokine concentrations and Up/Ucr were compared between disease categories using the Kruskal-Wallis test. Correlations between urinary chemokine levels and clinical and histological parameters were determined with the Spearman rank correlation coefficient. Differences at p value of < 0.05 were considered statistically significant.
Twenty-six children aged 4.2-15.5 (median 9.2) years were enrolled in this study. All patients underwent diagnostic renal biopsy and concurrent collection of urine samples. No patient had active infection at that time. The mean values for Up/Ucr, grade of OB and eGFR among the study participants were 2.10 ± 3.50, 1.9 ± 1.0 and 102.5 ± 28.9 ml/min/1.73 m2, respectively. The mean urinary fractalkine and MCP-1 concentrations were 3.89 ± 2.67 mg/gCre (range, 0.07-9.86), and 1.63 ± 2.76 mg/gCre (range, 1.70-10.96), respectively. As for histologic indices in patients with MesPGN, the mean AI and CI were 3.6 ± 1.8 (range, 2-7) and 3.1 ± 1.0 (range, 2-5), respectively. Clinical characteristics of the study patients in each disease category are shown in Table 1.
When the levels of Up/Ucr and urinary chemokine concentrations were compared between different categories of glomerular disease, only the urinary fraktalkine concentration differed significantly (p = 0.033) (Fig. 1). In contrast, no significant differences between the disease categories were seen in the urinary MCP-1 concentration and the Up/Ucr (p = 0.096 and p = 0.112, respectively) (Fig. 1). Although the urinary concentrations of fraktalkine and MCP-1 were correlated with each other (fraktalkine/cr vs. MCP-1/cr, r = 0.568, p = 0.002), neither was significantly correlated with the value of Up/Ucr (fraktalkine/cr vs. Up/Ucr, r = 0.339, p = 0.083, and MCP-1/cr vs. Up/Ucr, r = 0.065, p = 0.749, respectively). As for clinical parameters other than Up/Ucr, the concentrations of both urinary chemokines demonstrated a significant positive correlation with the grade of OB (fraktalkine/cr vs. the grade of OB, r = 0.689, p < 0.001, and MCP-1/cr vs. the grade of OB, r = 0.387, p = 0.046, respectively), and a significant inverse correlation with the value of eGFR (fraktalkine/cr vs. eGFR, r = 0.553, p = 0.003, and MCP-1/cr vs. eGFR, r = 0 .822, p < 0.001, respectively). As a result, an increased urinary fractalkine concentration was strongly associated with the severity of hematuria, whereas an increased urinary MCP-1 concentration was strongly associated with a decline of the eGFR (Fig. 2). With regard to histologic indices in patients with MesPGN, although the AI did not show a correlation with the concentration of each of the urinary chemokines (AI vs. fraktalkine/cr, r = 0.189, p = 0.517, and AI vs. MCP-1/cr, r = 0.391, p = 0.167, respectively), we confirmed a significant positive correlation between the CI and urinary MCP-1 concentration (r = 0.666, p = 0.009), whereas there was no correlation between the CI and urinary fraktalkine concentration (r = 0.0274, p = 0.926).

Clinical characteristics and laboratory findings of the study patients.
TBMD, thin basement membrane disease; IgAN, IgA nephropathy; MN, membranous nephropathy; LN, lupus nephritis; AGN, acute glomerulonephritis; GFR, glomerular filtration rate.

Comparison of urinary chemokine excretion from the diagnostic groups.
A significant difference in urinary fractalkine concentrations (A) was observed (Kruskal-Wallis test, p = 0.033), whereas there were no significant differences in the urinary concentrations of monocyte chemoattractant protein-1 (MCP-1) (B) and the urinary protein/creatinine ratio (C) (p = 0.096 and p = 0.112, respectively).
(TBMD, thin basement membrane disease; LN, lupus nephritis; IgAN, IgA nephropathy; MN, membranous nephropathy; AGN, acute glomerulonephritis).
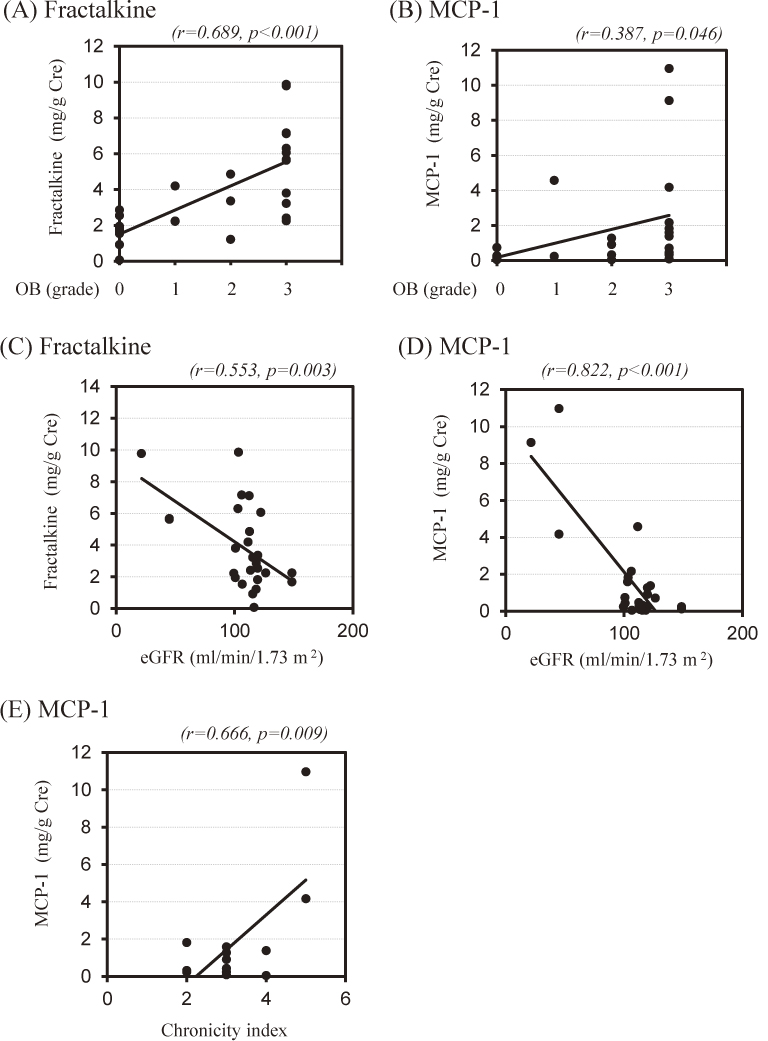
Correlation of urinary fractalkine or MCP-1 concentrations with the severity of glomerulonephritis.
Both urinary fractalkine and MCP-1 concentrations show a significant association with the grade of occult blood in urine (OB: grade 0, n = 7; grade 1, n = 3; grade 2, n = 4 and grade 3, n = 12) (A and B) and the value of the estimated GFR (eGFR: n = 26) (C and D). Note the strong association between urinary fractalkine concentration and OB (A, r = 0.689, p < 0.001), and between urinary MCP-1 concentration and eGFR (D, r = 0.822, p < 0.001). Urinary MCP-1 concentration also shows a significant association with the histologic chronicity indices (n = 18: 2 points, 5; 3 points, 9; 4 points, 2 and 5 points, 2) (E, r = 0.666, p = 0.009).
With regard to the origin of urinary chemokines, it has been reported that fractalkine and MCP-1 are expressed by the mesangial, podocyte and infiltrating mononuclear cells in response to pro-inflammatory signals (Watson and Beresford 2013). Thus, both residual glomerular cells and infiltrating cells may be attributable to the excretion of inflammatory chemokines into urine. However, little information is available regarding the implication of urinary proinflammatory chemokine levels in pediatric-onset renal diseases (Watson et al. 2012). So far, it has been reported that the urinary MCP-1 concentration may predict disease activity in adulthood IgAN and LN (Stangou et al. 2009, 2013; Abujam et al. 2013). Interestingly, Stangou et al. (2009) have reported that the urinary MCP-1 concentration is significantly correlated with the degree of interstitial fibrosis, which is an index reflecting the histological chronicity, in adult patients with IgAN. Furthermore, they observed a marked reduction of urinary MCP-1 concentration after immunosuppressive treatment in patients with IgAN (Stangou et al. 2013). On the other hand, fraktalkine/CX3CL1 is a chemokine that induces chemotaxis and activation of cells expressing its receptor, CX3CR1. It has been reported that glomerular fraktalkine expression and intrarenal accumulation of CX3CR1-positive cells play a pivotal role in the pathogenesis and development of inflammatory renal diseases (Anders et al. 2003; Aizawa-Yashiro et al. 2013). Recently, we reported that glomerular fraktalkine expression is involved in the pathogenesis and aggravation of childhood IgAN (Aizawa-Yashiro et al. 2013; Tanaka and Imaizumi 2013). Furthermore, Cox et al. (2012) have reported that increased glomerular expression of fraktalkine induces the onset of gross hematuria in adult patients with IgAN. However, the implications of both urinary fractalkine and MCP-1 concentrations in pediatric patients with GN remain to be determined. Here, therefore, we examined whether the urinary concentrations of fractalkine and MCP-1 reflect the disease activity of childhood GN.
We found the increase in the urinary concentration of fractalkine varied significantly between different types of GN, and that the urinary concentrations of both fractalkine and MCP-1 reflected disease activity, in terms of the grade of hematuria and the value of eGFR, although our results remain preliminary. Notably, relatively high levels of urinary concentrations of both fractalkine and MCP-1 were seen in patients with AGN who showed a marked decrease in the value of eGFR. This observation may further support above issue. Interestingly, we observed no significant correlation between Up/Ucr and the concentrations of either urinary chemokine in the study participants, despite the fact that urinary protein excretion is generally known to be a simple marker of disease activity in patients with glomerular diseases. Judging from their scores, their renal biopsy findings from most patients continued to suggest mild to moderate disease severity. We think this issue may have accounted for the discrepancy in the values of Up/Ucr and urinary chemokine concentrations, although this theory remains to be further determined. We believe that measurement of urinary fractalkine and MCP-1 concentrations in addition to Up/Ucr may have contributed to early detection of glomerular injury.
Interestingly, we confirmed a strong association between increased urinary fractalkine concentrations and the severity of hematuria. Based on recent reports regarding the implications of expression of glomerular fractalkine in patients with IgAN (Cox et al. 2012), fractalkine is thought to be a strong inducer of glomerular basement membrane (GBM) injury, resulting in massive hematuria. Thus, the urinary fractalkine concentration may be a predictor of inflammatory GBM damage in selected patients with GN. On the other hand, we confirmed a strong association between an increased urinary MCP-1 concentration and the value of eGFR combined with the histologic CI. Based on recent reports regarding the implications of the urinary MCP-1 concentration in adult patients with IgAN (Stangou et al. 2009, 2013), MCP-1 is thought to be a possible inducer of tubulointerstitial damage as well as glomerular injury, resulting in a decrease of renal function. However, these theories remain speculative, and further investigations are needed.
Since urinary proinflammatory chemokine excretion may reflect histologic inflammatory damage in patients with GN (Stangou et al. 2009, 2013; Watson et al. 2012; Abujam et al. 2013; Korzeniecka-Kozerska et al. 2013), measurement of urinary cytokine/chemokine concentrations may lead to the development future non-invasive procedures for prediction of disease activity in patients with GN, rather than the use of invasive renal biopsy (Stangou et al. 2009). We also have reported the usefulness of measuring the concentrations of urinary fractalkine and MCP-1 in children with GN, although we did not examine the implications of other reported urinary cytokines/chemokines such as interleukin-6, epidermal growth factor, and interferon-γ-inducible protein 10 (Stangou et al. 2009; Abujam et al. 2013). Thus, the most suitable urinary cytokine/chemokine for precisely reflecting the extent of glomerular inflammation in a routine setting remains to be determined. However, we believe that measurement of urinary fractalkine and MCP-1 concentrations in addition to Up/cr would be a useful non-invasive approach for prediction of glomerular inflammation in children with GN.
In this study, we first examined whether urinary concentrations of fractalkine and MCP-1 showed a significant differences between inflammatory kidney diseases and non-inflammatory kidney disease (thin basement membrane disease) in children. We found the possible usefulness of measurement of urinary concentrations of fractalkine and MCP-1 in children with various types of GN. However, we did not examine urinary concentrations of both chemokine in healthy children, since it was not possible to obtain the samples during study period. Further investigations of this issue are needed.
This work was supported by a grant for research 2012 from the Kampo-zaidan Foundation (Tokyo, Japan) to H.T.
The authors declare no conflict of interest.