Abstract
Controversy exists regarding the similarity between depression as seen in patients with epilepsy and in those with idiopathic major depression. The objective of this study was to examine whether anger is a distinctive feature of depression in epilepsy. Participants included 487 adult patients with epilepsy (study group) and 85 patients with idiopathic major depression according to Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria, and without other neurological complications (control group). All participants completed the Inventory of Depressive Symptomatology Self-Report (IDS-SR) and the Buss-Perry Aggression Questionnaire (BAQ). The IDS-SR is a self-report questionnaire that measures depression severity and assesses all symptoms of depression as defined by the DSM-IV. The BAQ is a self-rating scale designed for assessing aggression. After examining potential confounding factors (i.e., demographic and clinical variables) using a multivariate linear regression model, BAQ scores were compared between the study (n = 85) and control groups (n = 54) for patients with moderate or severe depression using established cut-off points (IDS-SR score > 25). BAQ scores were significantly higher in the study group (P = 0.009). Among the BAQ subscales, only anger showed a statistically significant difference (P = 0.013). Although a significant correlation was revealed between the IDS-SR and BAQ scores in the study group, no such correlation was found in the control group. Thus, anger might be a constituent component of depression among epilepsy patients, but not among idiopathic major depression patients.
Introduction
Based on their evaluation of mood symptoms in patients with epilepsy, Blumer and colleagues proposed “interictal dysphoric disorder” as an epilepsy-specific mood disorder (Blumer et al. 1995). Furthermore, Kanner and Palac (2000) have emphasized the clinical significance of this epilepsy-specific mood disorder, demonstrating that at least three quarters of epilepsy patients with depressive symptoms have non-clinical depression according to the Diagnostic and Statistical Manual of Mental Disorders Fourth Edition (DSM-IV) criteria, and that anhedonia without sadness or irritability may predominate. However, others refute the idea of an epilepsy-specific mood disorder and suggest that depressive symptoms in epilepsy are similar to those seen in patients without epilepsy (Standage and Fenton 1975; Dodrill and Batzel 1986; Metcalfe et al. 1988; Reilly et al. 2006). Overall, the prevailing idea is that the constellation of depressive symptoms in patients with epilepsy mostly overlaps with that observed among patients with idiopathic major depression (Lambert and Robertson 1999).
From a pharmacotherapeutic perspective, this presumed similarity has resulted in the widespread belief in the efficacy of antidepressants in epilepsy patients. Indeed, the safety of antidepressants—serotonin-reuptake inhibitors, in particular—among epilepsy patients has been widely demonstrated (Favale et al. 1995; Kanner et al. 2000; Hovorka et al. 2000; Kühn et al. 2003; Specchio et al. 2004; Thomé-Souza et al. 2007). However, efficacy has been successfully tested in only a handful of open, uncontrolled studies (Favale et al. 1995; Kanner et al. 2000; Hovorka et al. 2000; Kühn et al. 2003; Specchio et al. 2004; Thomé-Souza et al. 2007). The only double-blind, randomized controlled study conducted thus far failed to demonstrate the superiority of antidepressants over a placebo in patients with epilepsy, though the sample size was relatively small (Robertson and Trimble 1985). In contrast, mood stabilizers such as lamotrigine (LTG) have been shown to improve moods in patients with epilepsy in at least in 11 studies, including 3 randomized control investigations (Edwards et al. 2001; Sackellares et al. 2002; Kalogjera-Sackellares and Sackellares 2002; Kaminow et al. 2003; Cramer et al. 2004; Ettinger et al. 2007; Fakhoury et al. 2007, 2008; Labiner et al. 2009; Kato et al. 2011). Interestingly, Labiner et al. (2009) recently revealed that the effects of LTG on mood primarily affect anger or hostility rather than depression itself. Our recent findings support this effect (Kato et al. 2011). Thus, it seems likely that patients with epilepsy are characterized by a different constellation of depression symptoms—one in which anger or hostility is a distinctive feature. In the present study, we examined whether anger and hostility are actually distinctive features of depression in epilepsy in comparison with idiopathic major depression.
Methods
Data for the study group were prospectively collected from 487 adults who were treated as outpatients at Aichi Medical University, Hirosaki University, Shizuoka Institute of Epilepsy and Neurological Disorders, Musashino Kokubunji Clinic, and Tanaka Epilepsy Clinic from October 2, 2009 to April 1, 2011. In order to take part in the study group, participants were required to be older than 16 years of age, diagnosed with epilepsy by a board certified epileptologist (Japan Epilepsy Society), and have the capacity to consent to and complete questionnaires. Patients with suspected or confirmed non-epileptic seizures and frank psychosis were excluded. In order to take part in the control group, participants were required to be older than 16 years of age, and diagnosed with idiopathic major depression by a board certified psychiatrist (Japan Neuropsychiatric Society) according to the DSM-IV criteria. Patients with neurological disease, a serious physical condition such as cancer, personality or developmental disorders, post-traumatic stress disorder, psychosis, or bipolar disorder were excluded from the control group. All subjects gave written informed consent and complete questionnaires. This study was approved by the ethical committees of Aichi Medical University, Hirosaki University, and Shizuoka Institute of Epilepsy and Neurological Disorders.
Demographic and clinical information, including age, sex, seizure frequency, seizure subtype, age at time of first unprovoked seizure, medication history and psychiatric history, were recorded by epilepsy specialists who assisted with data collection. Information was also gathered from patient histories, examinations, and reviews of outpatient notes.
Of the 487 patients in the study group and 85 in the control group, 463 and 82, respectively, successfully completed the Japanese version of the Inventory of Depressive Symptomatology Self-Report (IDS-SR) and the Buss-Perry Aggression Questionnaire (BAQ). The IDS-SR (30 items) and BAQ (29 items) are widely used self-rating scales for measuring elements of depression and aggression, respectively (Rush et al. 1986; Buss and Perry 1992; Ando et al. 1999; Nakano 2001; Trivedi et al. 2004). The IDS-SR is a self-report questionnaire that measures depression severity and assesses all symptoms of depression as defined by the DSM-IV, including melancholic, atypical, and anxious symptoms. The BAQ is a self-rating scale designed for assessing aggression. It is based on a four-factor model consisting of instrumental, motor, emotional, and cognitive components, which enables phenomenological analysis of aggression in patients. Only patients with moderate or severe depression (IDS-SR score > 25) were included in this comparison, because mild depression is typically characterized by a more heterogeneous patient group than moderate or severe depression. On this criterion, 85 patients in the study group and 54 in the control group were included in the final analyses. A consort diagram is shown in Fig. 1.
Characterization of epilepsy type and localization of epileptic foci were assessed based on video-electroencephalogram (EEG) data when ictal recordings were available. When no such data were available, subjects with a clinical diagnosis of simple or complex focal seizures were considered to have focal epilepsy, whereas those with typical absence or generalized tonic-clonic seizures exclusively on awakening with or without generalized spike-and-wave complex, together with juvenile myoclonic epilepsy, were categorized as having idiopathic generalized epilepsy. Patients with only generalized events without any other accompanying seizures or any predilection for a particular time zone were categorized as having an unknown type of epilepsy. Those who showed evidence of bilateral involvement, either clinically, or from EEG or imaging findings, were judged as being bilaterally involved. Complete control of seizures, except for aura sensation, lasting for more than 1 year prior to this study was considered a state of being seizure free. If any seizure occurred within 1 month prior to the study, it was considered a recently preceding seizure.
Statistical analyses were performed using SPSS version 16.0, and consisted of two steps. First, multivariate regression models were used to evaluate the following potential determinants of the IDS-SR and BAQ scores: age, age at time of first unprovoked seizure, sex, presence/absence of recently preceding seizures, complex partial seizure or generalized events, epilepsy type (focal or idiopathic generalized), lobar localization (temporal vs. extra-temporal), seizure-free or experiencing active seizures, indications of bilateral involvement, administration of antidepressants, and the doses of 10 antiepileptic drugs (AEDs) prescribed in more than 1% of the patients with epilepsy. The variance inflation factors remained within 4 for all of the other variables. Next, BAQ scores were compared between groups. Then, using two-tailed, independent-groups t-tests and chi-square tests, we compared BAQ scores between groups, along with demographic data, the IDS-SR scores, and scores of four BAQ subscales (Anger, Hostility, Physical aggression, Verbal aggression). Correlation coefficients between BAQ and IDS-SR scores within each group were also examined.

Results
The main demographic and clinical characteristics of the subjects are listed in Table 1. Of 361 patients (78.0%) with focal epilepsy, lobar localization was determined in 319 [218 temporal (47.1%), 82 frontal (17.7%), 12 occipital (2.0%), 7 parietal (1.5%)]. Abnormal IDS-SR scores were recorded for 287 patients (62%), which were divided by mild (14-25, 36.7%), moderate (26-39, 18.2%), and severe (≥ 40, 7.1%) depression. Multivariate linear regression findings revealed that recent seizure (P = 0.007) and administration of zonisamide (P = 0.002) had a significant impact on IDS-SR score, while age (P = 0.013) was the only potential determinant of total BAQ score. No variance inflation factors for clinical variables exceeded 4.
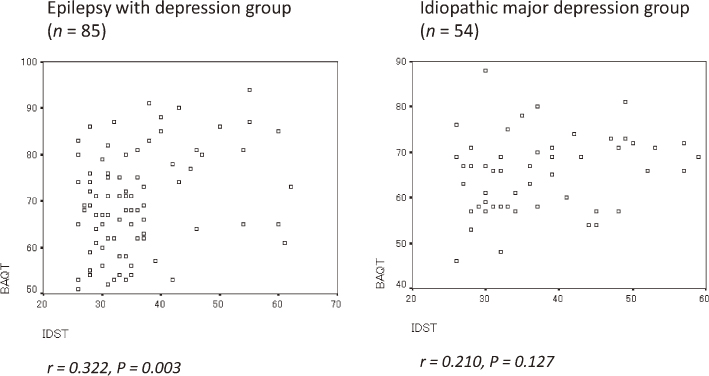
The main demographic and clinical characteristics of the study group are listed in Table 2. As illustrated in Table 3, BAQ scores were significantly higher in the study group than the control group (P = 0.008). Even after excluding patients given zonisamide, this difference remained statistically significant (P = 0.040). Among the BAQ subscales, anger was the only indicator that showed a statistically significant difference (P = 0.009) (Fig. 2). Fig. 3 presents a scatter plot for the IDS-SR and BAQ differences between the study and control groups. There was a significant positive correlation between IDS-SR and BAQ in the study group. There was no similar correlation observed in the control group.
Discussion
The present study revealed two important findings. First, although depressive patients with epilepsy and those with idiopathic major depression showed similarities in depressive score severity based on the IDS-SR, the two groups differed in awareness of aggressive mood, as demonstrated by the BAQ. Second, while aggressive tendencies were intrinsically related to depressive symptoms in the epilepsy group, depression and aggression appeared to be independent in the idiopathic major depression group. These findings taken together suggest that depressive and aggressive moods are closely tied among epilepsy patients with depressive symptoms. Thus, it appears that the two groups are characterized by different psychopathological features.
The prevalence of moderate or severe depressive states in the present sample (25.3%) is concordant with previous reports on prevalence, which range from 20% to 55% (Jacoby et al. 1996; O’Donoghue et al. 1999). Understandably, clinical variables that have been associated with depressive symptoms in epilepsy vary substantially due to sampling differences between studies (Harden 2002; Grabowska-Grzyb et al. 2006; Mensah et al. 2006). Nevertheless, our findings replicated some of the important associations between depressive symptoms and demographic and clinical variables among patients with epilepsy suggested in previous reports. Recent seizure, which was a potential determinant of depression in the present sample, was also described as such by Mensah and colleagues (Mensah et al. 2006). Depression among patients with recent seizure may be associated with postictal depression, given that interictal depression is intimately connected with postictal depression. This may at least partially explain the seemingly intermittent nature of depression in patients with epilepsy.
It is well known that virtually all AEDs are associated with depressive symptoms in patients with epilepsy, though some to a greater degree than others. Compounds such as phenobarbital, primidone, topiramate, and vigabatrin are often associated with depressive responses (McConnell and Duncan 1998; Lopez-Gomez et al. 2005). Even LTG, which is well known for its depressolytic properties, has been reported to be associated with depression in some cases (Kanner and Palac 2000). In the present population, only zonisamide was found to have a substantial impact on IDS-SR scores. However, results remained consistent regardless of whether patients given zonisamide were included or excluded.
It has been consistently noted that depressive disorders in epilepsy are under-recognized and, consequently, under-treated (Lambert and Robertson 1999; Kanner and Palac 2000; Schmitz 2005). Certainly, the frequent presence of depressive symptoms in epilepsy, which may be milder and more intermittent than those in patients with idiopathic major depression, may contribute to this under-recognition (Lambert and Robertson 1999; Kanner and Palac 2000; Schmitz 2005). However, even moderate or severe depressive states in patients with epilepsy that might lead to suicide attempts have apparently escaped medical attention. The unusual constellation of depressive symptoms, as demonstrated in the current sample, may lead to inconsistent clinical judgments among mental illness specialists, which may serve as a supplementary explanation for the lack of recognition of this serious problem.
Nevertheless, we must exercise caution to avoid the simplified and misleading conclusion that high BAQ scores are directly linked to violent behaviors. The BAQ, which was utilized in the current study, is a self-rating scale composed of 29 items and an acknowledged standard assessment tool for measurement of aggression (Nakano 2001). Factor analysis of the test items yielded four subscales: physical aggression, verbal aggression, anger, and hostility (Nakano 2001). Among the four subscales of the BAQ, verbal aggression and physical aggression represent the instrumental or motor component of behavior for the purpose of hurting and harming others (Jacoby et al. 1996). On the other hand, anger, which involves physiological arousal and preparation for aggression, represents the emotional or affective component of aggressive behavior. Hostility, which consists of feelings of malevolence and injustice, represents the cognitive component of behavior (O’Donoghue et al. 1999). In the present study, of the 4 BAQ subscales, only anger showed a significant increase in the study group as compared with the control group, and no statistically significant differences were found between the groups for verbal and physical aggression, which can be directly associated with violent behaviors.
It is now widely recognized that failure to recognize and treat depression in patients with epilepsy can result in serious consequences. However, it is conspicuous that a fundamental question, namely, whether antidepressants are as effective among patients with epilepsy as in those with idiopathic major depression, remains to be answered. It is known that antidepressants are less effective for the depressive phase of bipolar disorder than for the depressive state of idiopathic major depression (monopolar depression), whereas the reverse appears to be true for mood modulators such as LTG and olanzapine. In individuals affected by bipolar disorder, aggressive agitation, even in the midst of a depressive phase, has been reported (Maj et al. 2003). The prevalence of anger among patients with both depression and epilepsy observed in the present study strongly suggests that a re-examination of the therapeutic strategy for this population is necessary. Medication with anti-anger properties should take priority over antidepressants in the first therapeutic trial for some epilepsy patients with depression.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Ando,
A.,
Soga,
S.,
Yamasaki,
K.,
Shimai,
S.,
Shimada,
H.,
Utsuki,
N.,
Oashi,
O. &
Sakai,
A.
(1999) Development of the Japanese version of the Buss-Perry Aggression Questionnaire (BAQ). Shinrigaku Kenkyu, 70, 384-392.
-
Blumer,
D.,
Montouris,
G. &
Hermann,
B.
(1995) Psychiatric morbidity in seizure patients on a neurodiagnostic monitoring unit. J. Neuropsychiatry Clin. Neurosci., 7, 445-456.
-
Buss,
A.H. &
Perry,
M.
(1992) The aggression questionnaire. J. Pers. Soc. Psychol., 63, 452-459.
-
Cramer,
J.A.,
Hammer,
A.E. &
Kustra,
R.P.
(2004) Improved mood states with lamotrigine in patients with epilepsy. Epilepsy Behav., 5, 702-707.
-
Dodrill,
C.B. &
Batzel,
L.W.
(1986) Interictal behavioral features of patients with epilepsy. Epilepsia, 27 Suppl 2, S64-76.
-
Edwards,
K.R.,
Sackellares,
J.C.,
Vuong,
A.,
Hammer,
A.E. &
Barrett,
P.S.
(2001) Lamotrigine monotherapy improves depressive symptoms in epilepsy: a double-blind comparison with valproate. Epilepsy Behav., 2, 28-36.
-
Ettinger,
A.B.,
Kustra,
R.P. &
Hammer,
A.E.
(2007) Effect of lamotrigine on depressive symptoms in adult patients with epilepsy. Epilepsy Behav., 10, 148-154.
-
Fakhoury,
T.A.,
Barry,
J.J.,
Miller,
J.M.,
Hammer,
A.E. &
Vuong,
A.
(2007) Lamotrigine in patients with epilepsy and comorbid depressive symptoms. Epilepsy Behav., 10, 155-162.
-
Fakhoury,
T.A.,
Miller,
J.M.,
Hammer,
A.E. &
Vuong,
A.
(2008) Effects of lamotrigine on mood in older adults with epilepsy and co-morbid depressive symptoms: an open-label, multicentre, prospective study. Drugs Aging, 25, 955-962.
-
Favale,
E.,
Rubino,
V.,
Mainardi,
P.,
Lunardi,
G. &
Albano,
C.
(1995) Anticonvulsant effect of fluoxetine in humans. Neurology, 45, 1926-1927.
-
Grabowska-Grzyb,
A.,
Jedrzejczak,
J.,
Nagańska,
E. &
Fiszer,
U.
(2006) Risk factors for depression in patients with epilepsy. Epilepsy Behav., 8, 411-417.
-
Harden,
C.L.
(2002) The co-morbidity of depression and epilepsy: epidemiology, etiology, and treatment. Neurology, 59 Suppl 4, S48-55.
-
Hovorka,
J.,
Herman,
E. &
Nemcova,
I.
(2000) Treatment of interictal depression with citalopram in patients with epilepsy. Epilepsy Behav., 1, 444-447.
-
Jacoby,
A.,
Baker,
G.A.,
Steen,
N.,
Potts,
P. &
Chadwick,
D.W.
(1996) The clinical course of epilepsy and its psychosocial correlates: findings from a U.K. Community study. Epilepsia, 37, 148-161.
-
Kalogjera-Sackellares,
D. &
Sackellares,
J.C.
(2002) Improvement in depression associated with partial epilepsy in patients treated with lamotrigine. Epilepsy Behav., 3, 510-516.
-
Kaminow,
L.,
Schimschock,
J.R.,
Hammer,
A.E. &
Vuong,
A.
(2003) Lamotrigine monotherapy compared with carbamazepine, phenytoin, or valproate monotherapy in patients with epilepsy. Epilepsy Behav., 4, 659-666.
-
Kanner,
A.M.,
Kozak,
A.M. &
Frey,
M.
(2000) The use of sertraline in patients with epilepsy: is it safe? Epilepsy Behav., 1, 100-105.
-
Kanner,
A.M. &
Palac,
S.
(2000) Depression in epilepsy: a common but often unrecognized comorbid malady. Epilepsy Behav., 1, 37-51.
-
Kato,
H.,
Fukatsu,
N.,
Noguchi,
T.,
Oshima,
T.,
Tadokoro,
Y. &
Kanemoto,
K.
(2011) Lamotrigine improves aggression in patients with temporal lobe epilepsy. Epilepsy Behav., 21, 173-176.
-
Kühn,
K.U.,
Quednow,
B.B.,
Thiel,
M.,
Falkai,
P.,
Maier,
W. &
Elger,
C.E.
(2003) Antidepressive treatment in patients with temporal lobe epilepsy and major depression: a prospective study with three different antidepressants. Epilepsy Behav., 4, 674-679.
-
Labiner,
D.M.,
Ettinger,
A.B.,
Fakhoury,
T.A.,
Chung,
S.S.,
Shneker,
B.,
Tatum IV,
W.O.,
Mitchell Miller,
J.,
Vuong,
A.,
Hammer,
A.E. &
Messenheimer,
J.A.
(2009) Effects of lamotrigine compared with levetiracetam on anger, hostility, and total mood in patients with partial epilepsy. Epilepsia, 50, 434-442.
-
Lambert,
M.V. &
Robertson,
M.M.
(1999) Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia, 40 Suppl 10, S21-47.
-
Lopez-Gomez,
M.,
Ramirez-Bermudez,
J.,
Campillo,
C.,
Sosa,
A.L.,
Espinola,
M. &
Ruiz,
I.
(2005) Primidone is associated with interictal depression in patients with epilepsy. Epilepsy Behav., 6, 413-415.
-
Maj,
M.,
Pirozzi,
R.,
Magliano,
L. &
Bartoli,
L.
(2003) Agitated depression in bipolar I disorder: prevalence, phenomenology, and outcome. Am. J. Psychiatry, 160, 2134-2140.
-
McConnell,
H.W. &
Duncan,
D.
(1998) Behavioral effects of antiepileptic drugs. In Psychiatric Co-Morbidity in Epilepsy: Basic Mechanisms, Diagnosis, and Treatment, edited by
McConnell,
H.W. &
Snyder,
P.J.
American Psychiatric Press, Washington, DC, pp. 335-370.
-
Mensah,
S.A.,
Beavis,
J.M.,
Thapar,
A.K. &
Kerr,
M.
(2006) The presence and clinical implications of depression in a community population of adults with epilepsy. Epilepsy Behav., 8, 213-219.
-
Metcalfe,
R.,
Firth,
D.,
Pollock,
S. &
Creed,
F.
(1988) Psychiatric morbidity and illness behaviour in female neurological in-patients. J. Neurol. Neurosurg. Psychiatry, 51, 1387-1390.
-
Nakano,
K.
(2001) Psychometric evaluation on the Japanese adaptation of the Aggression Questionnaire. Behav. Res. Ther., 39, 853-858.
-
O’Donoghue,
M.F.,
Goodridge,
D.M.,
Redhead,
K.,
Sander,
J.W. &
Duncan,
J.S.
(1999) Assessing the psychosocial consequences of epilepsy: a community-based study. Br. J. Gen. Pract., 49, 211-214.
-
Reilly,
R.E.,
Bowden,
S.C.,
Bardenhagen,
F.J. &
Cook,
M.J.
(2006) Equality of the psychological model underlying depressive symptoms in patients with temporal lobe epilepsy versus heterogeneous neurological disorders. J. Clin. Exp. Neuropsychol., 28, 1257-1271.
-
Robertson,
M.M. &
Trimble,
M.R.
(1985) The treatment of depression in patients with epilepsy: a double-blind trial. J. Affect. Disord., 9, 127-136.
-
Rush,
A.J.,
Giles,
D.E.,
Schlesser,
M.A.,
Fulton,
C.L.,
Weissenburger,
J. &
Burns,
C.
(1986) The inventory for depressive symptomatology (IDS): preliminary findings. Psychiatry Res., 18, 65-87.
-
Sackellares,
J.,
Kwong,
J.,
Vuong,
A.,
Hammer,
A.E. &
Barrett,
P.S.
(2002) Lamotrigine monotherapy improves health-related quality of life in epilepsy: a double-blind comparison with valproate. Epilepsy Behav., 3, 376-382.
-
Schmitz,
B.
(2005) Depression and mania in patients with epilepsy. Epilepsia, 46 Suppl 4, 45-49.
-
Specchio,
L.M.,
Iudice,
A.,
Specchio,
N.,
La Neve,
A.,
Spinelli,
A.,
Galli,
R.,
Rocchi,
R.,
Ulivelli,
M.,
de Tommaso,
M.,
Pizzanelli,
C. &
Murri,
L.
(2004) Citalopram as treatment of depression in patients with epilepsy. Clin. Neuropharmacol., 27, 133-136.
-
Standage,
K.F. &
Fenton,
G.W.
(1975) Psychiatric symptom profiles of patients with epilepsy. A controlled investigation. Psychol. Med., 5, 152-160.
-
Thomé-Souza,
M.S.,
Kuczynski,
E. &
Valente,
K.D.
(2007) Sertraline and fluoxetine: safe and effective treatments for children and adolescents with epilepsy and depression. Epilepsy Behav., 10, 417-425.
-
Trivedi,
M.H.,
Rush,
A.J.,
Ibrahim,
H.M.,
Carmody,
T.J.,
Biggs,
M.M.,
Suppes,
T.,
Crismon,
M.L.,
Shores-Wilson,
K.,
Toprac,
M.G.,
Dennehy,
E.B.,
Witte,
B. &
Kashner,
T.M.
(2004) The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: a psychometric evaluation. Psychol. Med., 34, 73-82.