2014 年 232 巻 2 号 p. 137-144
2014 年 232 巻 2 号 p. 137-144
Coronary artery ectasia (CAE) is characterized by inappropriate dilation of the coronary vasculature. The underlying mechanisms of CAE formation are not yet entirely known. A polymorphism in the endothelial nitric oxide synthase (eNOS) gene, which reduces eNOS activity, might be a risk factor for coronary heart diseases. However, its role in CAE is unknown. One of the most studied eNOS gene polymorphisms is a c.894G>T polymorphism that results in the conversion of Glu (GAG) to Asp (GAT) at position 298. In this study, we investigated the potential association between the c.894G>T (Glu298Asp) polymorphism and CAE. The present study included 84 subjects from 2,980 consecutive patients in whom elective diagnostic coronary angiography was performed. Forty patients with isolated CAE and 44 subjects with normal coronary arteries were enrolled. The frequencies of the G allele were 78.4% in the control and 57.5% in CAE patients. The TT genotype was more frequent in patients with CAE than that in the controls (20% vs. 4.5%, p = 0.013). Furthermore, the risk of developing CAE in the presence of the homozygous TT genotype was significantly higher in the patients than that in the controls (OR = 7.7, 95% CI = 1.44-41.3). The presence of an 894T allele increased the risk of CAE 2.8-fold (95% CI = 1.15-6.73; p = 0.027). The frequencies of the T allele were 65% in CAE patients and 38.6% in the controls. In conclusion, the c.894G>T polymorphism in the eNOS gene may be a risk factor for CAE.
Coronary artery ectasia (CAE) is a rare clinical entity and a well-known pathological disease of the coronary arteries. It is a specific form of atherosclerotic coronary artery disease (CAD) and is characterized by inappropriately localized or diffuse (affecting the entire length of artery) dilation of the coronary vasculature. The incidence of CAE in coronary angiographic series ranges from 0.3 to 5.3% (Markis et al. 1976; Swaye et al. 1983; Demopoulos et al. 1997; Satran et al. 2005; Manginas and Cokkinos 2006). The main coronary angiographic characteristics of CAE are impaired coronary blood flow, delayed antegrade coronary dye filling, segmental back flow phenomenon (milking phenomenon) and stasis with local deposition of dye in dilated coronary segments (Krüger et al. 1999). The diameter of coronary arteries widens more than 1.5 times in relation to that of the adjacent normal coronary vascular networks in this pathological entity, which results from the disruption of internal and external elastic laminae, with concomitant damage to the musculoskeletal structure (Swaye et al. 1983). The disease may be either congenital or acquired. Determining the severity of CAE is based on the involvement of a single vessel or multi-vessels (Markis et al. 1976). In most cases, CAE is found to coexist with CAD, whereas in 10-20% of cases, it is associated with inflammatory diseases or connective tissue disorders such as systemic lupus erythematosus, Marfan syndrome, and Ehlers-Danlos syndrome (Antoniadis et al. 2008). Accordingly, patients suffering from CAE often present with atherosclerotic heart disease-like symptoms, or conversely, they can be totally asymptomatic. About 50% of CAE patients present with atypical anginal pain, as the ectatic lesions can interfere with the coronary blood flow. CAE may cause myocardial ischemia or myocardial infarction without significant coronary artery stenosis due to intracoronary thrombosis within the ectatic segment and distal embolisation of this thrombotic material (Hartnell et al. 1985). However, the clinical importance and pathophysiology of CAE are not yet fully understood.
Nitric oxide (NO) is a potent vasodilator released by the endothelium, as well as by platelets and vascular smooth muscle cells. Although it has a very short half-life, it plays important roles in protecting the cardiac vascular network against myocardial damage by inhibiting platelet aggregation, proliferation of vascular smooth muscle cells and leukocyte adhesion to the vascular endothelium (Calvert 2011). Endothelial NO is synthesized by the enzyme endothelial nitric oxide synthase (eNOS), which is encoded by the gene located on chromosome 7q35-q36 (Marsden et al. 1993). Some single nucleotide polymorphisms, including T-786C, c.894G>T (Glu298Asp) and a variable-number tandem repeat (VNTR) in intron 4 (intron 4 a/b VNTR polymorphism), have been identified in the eNOS gene (Wang et al. 1996; Hingorani et al. 1999; Nakayama et al. 2000). NO production can be influenced by polymorphisms of the eNOS gene. It was previously shown that some polymorphisms in this gene that reduce the eNOS activity might be a risk factor for atherosclerotic heart disease. (Hibi et al. 1998; Nakayama et al. 2000).
This study aimed to investigate whether there is any association between the c.894G>T polymorphism in the eNOS gene (rs1799983) and CAE, and to determine the possible interactions between this polymorphism and other risk factors associated with CAE. The c.894G>T polymorphism results in the conversion of glutamic acid (GAG) to an aspartic acid (GAT) at position 298 of the eNOS protein.
Eighty-four Turkish Caucasian subjects out of 2,980 patients in whom elective diagnostic coronary angiographies were performed at the Istanbul Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital between June 2011 and May 2012 were included in the study. The study group comprised 40 patients with CAE and without any atherosclerotic heart disease. Patients who developed secondary coronary aneurysms following balloon angioplasty, coronary stent placement, brachytherapy, or atherectomy and who were diagnosed with vascular ectasia associated with Kawasaki disease or any known collagen vascular diseases were excluded. Patients who developed myocardial infarction or who had a coronary artery bypass grafting surgery were also excluded from this study. The control group consisted of 44 subjects who underwent coronary angiography and were found to be free of CAE with normal coronary arteries.
Data on all subjects were obtained from the patients’ medical files and were recorded in a database. These collected data included patient characteristics such as age, gender, and a brief medical history covering comorbid diseases, risk factors for coronary heart disease, the baseline physical examination findings, and laboratory parameters consisting of complete blood counts, serum lipid levels, electrolyte levels, and parameters indicative of liver and renal functions. CAE was classified according to the classification system proposed by Markis et al. (1976). Informed consent was obtained from all participants and the study protocol was approved by the Mehmet Akif Ersoy Thoracic and Cardiovascular Surgery Training and Research Hospital ethics committee.
Biochemical analysisBlood samples from a peripheral vein were taken to measure complete blood counts, serum lipid levels, other biochemical parameters, and DNA extraction at the time of admission to the hospital. Blood samples from all patients were collected after overnight fasting. Biochemical profiles were measured with a Cobas-C 501 (Roche Diagnostics, Mannheim, Germany) biochemical analyzer using Roche kits. Complete blood counts were measured from venous blood samples collected in K3 EDTA tubes using Mindray BC-5800 (Mindray Bio-Medical Electronics Co., Ltd., Shenzhen, China) device by the optical laser method.
Coronary angiography and transthoracic echocardiographyCoronary angiography was performed by the Judkins technique through femoral artery access. Coronary angiograms were analyzed by two experienced interventional cardiologists without knowledge of the laboratory measurements or the clinical status of the participants. CAE was defined as the segmental or diffuse dilation of the coronary arteries to more than 1.5-fold of the diameter of the adjacent segments of the same artery or of different arteries. The classification of CAE defined by Markis et al. (1976) was graded as follows: type 1, diffuse ectasia of ≥ 2 coronary arteries; type 2, diffuse ectasia in one coronary artery and localized ectasia in another coronary artery; type 3, diffuse ectasia of one coronary artery; and type 4, localized or segmental ectasia of only one coronary artery. Transthoracic echocardiography was performed on patients before discharge using a system V (Vingmed, GE, Horten, Norway) with a 2.5 MHz phased-array transducer. The left ventricular ejection fraction was measured using the modified Simpson’s rule (Schiller et al. 1989).
DNA extraction and the detection of genetic polymorphismGenomic DNA was extracted from whole blood samples in EDTA-containing tubes using a commonly applied spin column method (Abdel-Aziz and Mohamed 2013). The blood samples collected for genotypic determination were stored in 4°C until DNA extraction for up to 24 h. Genomic DNA was isolated from peripheral blood leukocytes using QIAamp DNA Blood Mini Kit (Qiagen GmbH, Hilden, Germany). The detection of the c.894G>T (Glu298Asp) polymorphism (rs1799983) in the eNOS gene was achieved by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP). Briefly, specific primer pairs were used to amplify a part of the eNOS gene containing the exon 7 by PCR with the following flanking intronic primers: (sense) 5′-CATGAGGCTCAGCCCCAGAAC-3′ and (antisense) 5′-AGTCAATCCCTTTGCGCTCAC-3′. The PCR amplification was performed in a 50-μL reaction mixture containing 10 μL genomic DNA, 30 μL one step PCR mixture [1 unit Taq polymerase, 10 mM KCl, 10 mM (NH4)2SO4, 20 mM Tris-HCl (pH 8.75), 0.1% Triton X-100, 0.1 mg/mL bovine serum albumin (BSA) and 200 nM dNTPs], 2 μL of each primer (BioBasic Inc., Ontario, Canada), and 8 μL distilled H2O. PCR was performed by denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 30 s, repeated for 30 cycles. Thereafter, digestion of the amplified DNA was performed using Mbo I restriction endonuclease. Only in the presence of a thymine nucleotide at position 894 (corresponding to Asp 298), but not in the absence of it (wild type), the resulting 206 bp PCR product was cleaved into two smaller fragments of 119 and 87 bp. Digestion fragments were then resolved by electrophoresis on a 2.5% agarose gel stained with ethidium bromide.
Statistical analysisAll statistical analyses were performed using the Number Cruncher Statistical System (NCSS) 2007 Statistical Software Pocket Program (Utah, USA). All p values were two-tailed. A p value of less than 0.05 was considered statistically significant. The mean and standard deviation or median and interquartile ranges were used for descriptive statistics. The normality of continuous variables was tested using the one-sample Kolmogorov-Smirnov Test. Differences between the categorical variables were determined by the Chi-square test. Analyses of the comparison between the nonparametric continuous variables were performed using the nonparametric Mann-Whitney U test. Comparisons between the means of two groups were performed using the Student’s t test. The associations between the categorical data and the Glu298Asp genotypes were investigated using the Chi-square test. Univariate and multivariate regression analyses were used to investigate the independent predictors of CAE.
The total population of this study consisted of 40 patients with CAE in the absence of any atherosclerotic heart disease (study group) and 44 patients with angiographically normal coronary arteries (control group). The baseline patient characteristics are presented in the Table 1. Although the mean age of the subjects in the control group was higher than that in the study group, the difference was not statistically significant (58.6 ± 10.3 vs. 55.4 ± 9.5 years; p = 0.146). The distributions of other characteristics such as gender, body mass indices, frequency of diabetes mellitus, smoking, and alcohol, as well as family history of CAD were similar between the two groups. Moreover, laboratory findings such as total cholesterol, low-density lipoprotein cholesterol, triglyceride, serum biochemistry findings, complete blood counts, uric acid, thyroid-stimulating hormone levels, and left ventricle ejection fractions were not significantly different between the study and the control groups. High-density lipoprotein (HDL) cholesterol levels were significantly lower in patients with ectasia than in the control patients (Table 2). In multivariate logistic regression analysis HDL was found to be weakly associated with CAE development (OR = 0.94, 95% CI = 0.89-0.99).
The most commonly involved artery was the right coronary artery (n = 26, 65%). The other commonly affected arteries were the left anterior descending artery (60%), the circumflex artery (55%), and the left main coronary artery (12.5%). Diffuse ectatic involvement of at least one coronary artery was found in 23 patients (57.5%). In the other 17 patients (42.5%), focal ectasia on a coronary artery was present. The patients with CAE were categorized according to the classification system proposed by Markis et al. (1976), which is based on the appearance and number of vessels involved. According to this classification, 17 (42.5%) patients were categorized into type 4 disease, whereas 8 (20%) patients had type 1, 9 (22.5%) had type 2, and 6 (15%) patients had type 3 disease.
The polymorphism status in the eNOS gene of the subjects with CAE and normal coronary arteries was investigated. Genotypes and allelic frequencies of the patients are demonstrated in Table 3. Twenty-seven (61.4%) of the patients in the control group were homozygous for the guanine nucleotide (GG genotype), whereas 15 (34.1%) were heterozygous for the GT genotype, and 2 (4.5%) were homozygous for the thymine (TT) genotype. The distribution of genotypes in the control did not differ significantly from that expected under Hardy-Weinberg equilibrium. In contrast, 14 (35%) patients in the study group were homozygous for the guanine nucleotide (GG genotype), whereas 18 (45%) were heterozygous for the GT genotype and 8 (20%) were homozygous for the TT genotype. The TT genotype was more frequent in patients with CAE compared to those with normal coronary arteries (20% vs. 4.5%, respectively; p = 0.013). Furthermore, the risk of developing CAE in patients with the TT genotype was significantly higher in the CAE group than the control group (OR = 7.7, 95% CI = 1.44-41.3). The presence of the T allele increased the risk of CAE 2.8-fold (95% CI = 1.15-6.73; p = 0.027). The frequency of the T allele was 65% in patients with CAE, in contrast to 38.6% in the control group (Table 3). However, the c.894G>T eNOS gene polymorphism was not an independent predictor of CAE in multivariate logistic regression analysis (Table 4).
In patients with CAE, there was no significant association between the polymorphism of the eNOS gene and the number of ectatic coronary arteries. The distribution of the genetic polymorphism was also similar within the subgroups categorized according to the classification system proposed by Markis et al. (1976) (Table 5).

The baseline patient characteristics.
Data are presented as mean ± standard deviation or n (%), where appropriate.
BMI, body mass index; CAD, coronary artery disease.

The baseline laboratory parameters of the study subjects.
Data are presented as mean ± standard deviation.
HDL, high-density lipoprotein; LDL, low-density lipoprotein; TSH, thyroid stimulating hormone.

The distribution of genotypes and allele frequencies within the study groups.
Data are presented as n (%).
CI, confidence interval; OR, Odds ratio.
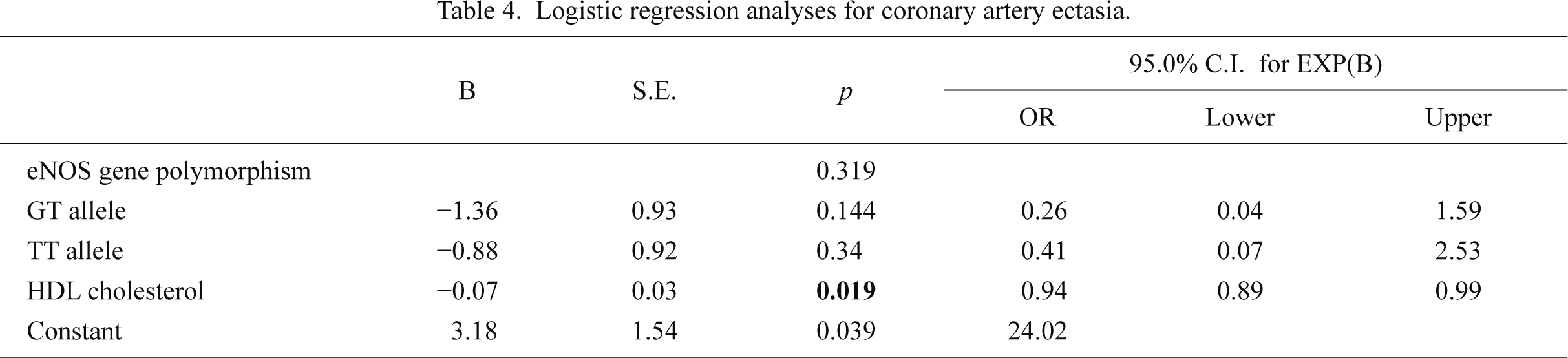
Logistic regression analyses for coronary artery ectasia.
CI, confidence interval; eNOS, endothelial nitric oxide synthase; HDL, high density lipoprotein; OR, Odds ratio.

Endothelial nitric oxide synthase gene polymorphism of the patients, the number of patients in each subcategory proposed by Markis et al. (1976), and the number of ectatic coronary arteries.
CAE, coronary artery ectasia; G, guanine; T, thymine.
Data are presented as n (%).
CAE is accidentally diagnosed in 1-5% of patients during coronary angiography (Swaye et al. 1983; Hartnell et al. 1985). It can be observed either in a diffuse form, affecting the total length of a coronary artery, or in a partial, localized form. Ectasia exists together with CAD in majority of CAE patients, and the right coronary artery is most commonly involved. All coronary arteries can be involved in a CAE case, but in 75% of the patients, CAE affects only a single coronary artery (Daoud et al. 1963; al-Harthi et al. 1991).
Endothelial NO, which is synthesized in endothelial cells from eNOS, inhibits platelet adhesion and aggregation, as well as promoting the vasodilatation of arterial and venous vessels. However, dysfunctional eNOS or its reduced activity has been shown to either initiate or accelerate atherogenesis (Li and Förstermann 2013). Genetic polymorphisms are known to modulate the functions of a gene product, but it is not yet clear how or which genetic polymorphisms affect eNOS activity, thereby resulting in reduced NO bioavailability. Salimi et al. (2012) also investigated the association between eNOS gene polymorphisms and the risk of CAD and plasma NO levels. They indicated that both the plasma NO levels and the frequency of T-786C gene polymorphism were higher in patients with CAD than the study controls. Tangurek et al. (2005) investigated the association of eNOS gene polymorphism (T-786C) with CAD in a Turkish population. The frequency of the C (cytosine) allele was found to be significantly higher in CAD.
The association of eNOS gene polymorphisms with the development and progression of CAD has been evaluated in several studies. Narne et al. (2013) investigated the effects of T-786C polymorphism in the eNOS gene on CAD in patients with diabetes. The frequencies of TC and CC genotypes, as well as the presence of the -786C allele, were found to be significantly higher in patients with CAD, especially with triple-vessel disease. However, the investigators could not demonstrate a similar relationship between the c.894G>T and 4a/b VNTR polymorphisms and CAD in patients with type 2 diabetes mellitus. Similarly, Saini et al. (2012) evaluated the effects of eNOS Glu298Asp polymorphism on Indian CAD patients. In their study, the frequency of the G allele was 87.5% in the patient group and 94% in the control group, while the presence of the T allele was found to be associated with CAD. In a recent study, the frequency of the TT genotype was shown to be significantly higher in patients with CAD in comparison to the control group (23% vs. 8%) (Syed and Jamil 2010). The researchers found that the G allele frequency was 60.2% for CAD patients and 76.6% for controls. These results are similar to those of our study, in which the G allele frequencies in the study group and the control group were 57.5% and 78.4%, respectively.
The presence of a polymorphic eNOS gene may be a risk factor for CAD. However, especially when the disease’s multifaceted presentation is considered, a single polymorphism alone may not be responsible for its etiology. Although several polymorphisms and/or mutations have been identified, the exact polymorphism that accounts for the etiology of CAD remains unclear.
To our knowledge, this study is the first where the association between the c.894G>T (Glu298Asp) polymorphism in the eNOS gene and the risk of CAE was evaluated. Herein, it was shown that the right coronary artery was the most commonly affected by ectasia. Diffuse ectatic involvement was found in 57.5% of the cases. In addition, we observed that TT homozygosity was significantly more frequent in patients with CAE than those with normal coronary arteries, and the risk of CAE was 7.7-fold higher in the TT genotype of the eNOS gene.
The c.894G>T polymorphism in the eNOS gene has been explored in several studies on CAD, but the present study is the first to that investigate the polymorphism in patients with CAE. In a previous study, the presence of the TT genotype was suggested to be an independent risk factor for premature CAD in Egyptians (Abdel-Aziz and Mohamed 2013). In this study, the TT genotype frequency was 19% in premature CAD patients and 10.1% in control subjects. The same gene polymorphism was also demonstrated to be associated with an increased risk for the development of myocardial infarction (Shimasaki et al. 1998; Antoniades et al. 2005). In Japanese patients, Shimasaki et al. (1998) found that the G allele frequency was 93.2% in the control group, while it was 89.3% in CAD patients. Our data on the G allele frequency were dissimilar to those for the Japanese population. The difference in the G allele frequency among various studies incorporating different races can be explained through ethnic diversity. In contrast, it was reported in a number of studies from different countries that the eNOS gene c.894G>T (Glu298Asp) polymorphism was not associated with an increased risk for CAD (Granath et al. 2001; Wang et al. 2001; Aras et al. 2002). Nassar et al. (2001) also reported that their results did not support the conclusion that the eNOS Asp298 allele contributes to the development of premature CAD. However, a meta-analysis including 23,028 patients suggested that homozygosity for the T allele is associated with an increased risk of ischemic heart disease (Casas et al. 2004). In that meta-analysis, the researchers observed significant differences in the frequency of the T allele by ethnic group. The T allele frequency was 7.6% in Asians versus 32.3% in non-Asians. In our study, frequencies of the T allele in CAE patients and control subjects were 42.5% and 21.6%, respectively.
The c.894G>T polymorphism has been investigated among the Turkish population. Cam et al. (2005) studied c.894G>T polymorphism in premature CAD patients. They found a significant association between the TT genotype and premature CAD. The G allele frequencies in healthy controls and in the patient group were 83.1% and 54.4%, respectively. Similarly, Aras et al. (2002) studied the same polymorphism in CAD, showing that the G allele frequencies were 71.8% in healthy controls and 64.7% in CAD patients. The results of our study and other previous studies on Turkish subjects show similar allele frequencies.
Several diseases, such as atherosclerosis or hypertension, are associated with impaired eNOS activity, as well as endothelial dysfunction and damage. The impaired expression and function of eNOS may severely affect endothelial integrity and function through reduced NO synthesis, leading to pathological disease states. Apart from hormones, stress, and hypoxia, eNOS gene polymorphisms may also result in abnormal eNOS expression and activity, which may be associated with increased oxidative stress, endothelial dysfunction, and increased inflammatory response (Albrecht et al. 2003). Sezen et al. (2010) investigated the association between oxidative stress and CAE. They observed that total anti-oxidant status decreased in patients with CAE and demonstrated that the total oxidant status and the oxidative status index values were significantly higher in the study group than in the control group. Due to the endothelial-protective function of reduced oxidative stress, increased expression of eNOS may help to ameliorate endothelial dysfunction-associated diseases such as CAD or CAE. Accordingly, Cheang et al. (2011) showed that increasing NO synthesis by directly enhancing the expression and activity of eNOS significantly reduced oxidative stress and improved endothelial dysfunction. Several factors contribute to the development of CAE, including vascular smooth muscle proliferation and migration, oxidative stress, increased inflammatory response, and abnormal collagen synthesis. In contrast, NO may protect against oxidative stress, endothelial dysfunction, and inflammation, which are responsible for the pathogenesis of CAE. Moreover, higher NO levels may also inhibit vascular smooth muscle cell proliferation and inflammation. Therefore, increased bioavailability of NO may also play an important role in preventing the development of CAE.
CAE can be accepted as a kind of atherosclerotic CAD; therefore, concerning our results, it is not surprising to find an association between low HDL levels and CAE development. In light of the data from this study, it would not be erroneous to suggest that the polymorphism in the eNOS gene may play a role in the pathogenesis of CAE and that a given eNOS gene polymorphism might be an important risk factor in the development of CAE. However, clinicians should take several important considerations into account when interpreting data from various studies. First, the distribution of eNOS gene polymorphisms may differ in populations from different ethnicities. Second, study groups should be well balanced in the design of the study, especially in terms of a number of risk factors such as ethnicity, environmental risk factors, and serum lipid levels. Researchers should also keep in mind that more than one polymorphism can exist in study subjects, and thus, determining and explaining the exact polymorphism that is responsible for the increased risk can be very problematic. Finally, the appropriate sample size for such a clinical study is debatable.
In conclusion, the results of our study indicate that the c.894G>T polymorphism in the eNOS gene may be a risk factor for CAE. These results provide the rationale for further studies with larger sample sizes that investigate the relationship between gene polymorphisms and CAE.
The authors declare no conflict of interest.