Abstract
Influenza vaccination is considered the single most important medical intervention for the prevention of influenza. The dose of trivalent influenza vaccine in children was increased almost double since 2011/12 season in Japan. We estimated the influenza vaccine effectiveness for children 1-11 years of age using rapid test kits in Isahaya City, involving 28,884 children-years, over two consecutive influenza seasons (2011/12 and 2012/13). Children were divided into two groups, vaccinated and unvaccinated, according to their vaccination record, which was obtained from an influenza registration program organized by the Isahaya Medical Association for all pediatric facilities in the city. There were 14,562 and 14,282 children aged from 1-11 years in the city in 2011 and 2012 respectively. In the 2011/12 season, the overall vaccine effectiveness in children from 1-11 years of age, against influenza A and B were 23% [95% confidence interval (CI): 14%-31%] and 20% [95% CI: 8%-31%], respectively. In the 2012/13 season, vaccine effectiveness against influenza A and B was 13% (95% CI: 4%-20%) and 9% (95% CI: −4%-21%), respectively. The vaccine effectiveness was estimated using the rapid diagnosis test kits. Age-stratified estimation showed that vaccine effectiveness was superior in younger children over both seasons and for both virus types. In conclusion, the trivalent influenza vaccine has a significant protective effect for children 1-11 years of age against influenza A and B infection in the 2011/12 season and against influenza A infection in the 2012/13 season in a community in Japan.
Introduction
Influenza virus is responsible for acute respiratory infections in every winter, and it is an important cause of morbidity and mortality. Epidemic resulted in approximately 3-5 million cases of severe illnesses and 250-500 thousand deaths (World Health Organization 2009). Vaccination is considered as the single most important medical intervention for prevention of influenza (Palache 2011; Centers for Disease Control and Prevention 2012).
In general, vaccine efficacy/effectiveness is expressed as a proportionate reduction in the disease attack rate between the vaccinated and unvaccinated study cohorts, and it can be calculated from the relative disease risk among the vaccinated group (Weinberg and Szilagyi 2010). Vaccine efficacy is best measured by double-blind, randomized controlled clinical trials, providing “best case scenarios” of vaccine protectiveness under controlled conditions and are commonly required before a new vaccine is licensed by the Food and Drug Administration and other global regulatory authorities (Clemens et al. 1996). The advantages of a vaccine efficacy study include rigorous control for biases afforded by randomization as well as prospective and active monitoring for disease attack rates and careful tracking of the vaccination status. In contrast, vaccine effectiveness is a “real world” view of how a vaccine actually reduces disease in a population. A study design capable of measuring vaccine effectiveness in this way is the “indirect cohort” or “quasi-cohort” study, in which different responses in the same vaccinated population are examined (Clemens and Shapiro 1984). The other option is “case-cohort” method, in which vaccination rates among cases are compared with those in a similar cohort as well as ecologic or observational studies. A meta-analysis of the Cochrane Database showed that vaccine efficacy of the trivalent inactivated vaccine against laboratory-confirmed influenza in healthy children is approximately 59% (Jefferson et al. 2012). Influenza vaccine efficacy/effectiveness considerably varies according to the time, place, and the degree of antigenic distance between the vaccine and circulating strains in each season (Osterholm et al. 2012). Therefore, it is necessary to assess vaccine efficacy/effectiveness in every season, at each study site.
The doses of trivalent vaccine used in Japan before the 2010/11 season were two doses of 0.1 ml for children less than 1 year of age, two doses of 0.2 ml for 1-5 years, two doses of 0.3 ml for 6-12 years, and a dose of 0.5 ml for 13 years and over. However, the amount was smaller than that is administered in the US or Europe, and thus, it was disputable whether such a low dose leads to sufficient seroresponse (Ochiai et al. 2009). Accordingly, from the 2011/12 season, the recommended dose of trivalent inactivated influenza vaccine for children was increased almost double to two doses of 0.25 ml for 6-24 months of age and two doses of 0.5 ml for 3-12 years, which met the recommendations of the Centers for Disease Control and Prevention’s Advisory Committee on Immunization Practices and the American Academy of Pediatrics recommendations (Health Service Bureau, Ministry of Health, Labour and Welfare 2011). However, there was no report estimating the influenza vaccine efficacy/effectiveness following the increase in the vaccine dosage for children in Japan.
In Isahaya City, Nagasaki Prefecture, Japan, the local government supports the expense of seasonal influenza vaccination for children less than 13 years of age every year. At the same time, Isahaya Medical Association conducts active influenza case surveillance and registration of all patients diagnosed as influenza using rapid test kits at nearly 80 internal medicine and pediatric facilities. In Japan, the rapid diagnostic test for influenza has been effectively used in routine medical practice. Rapid influenza diagnostic test has a high overall sensitivity of 82.8%-96.8% and specificity of 95.3%-97.6% (Kubo et al. 2003; Mitamura et al. 2004).
In the present study, we estimated vaccine effectiveness of influenza during the two influenza seasons following the introduction of the higher trivalent influenza vaccine dose for children, and assessed the protective effect of the vaccine against influenza A and B in a community in Japan.
Methods
Study site
The study was conducted in Isahaya City in Nagasaki Prefecture, located in southwestern Japan. According to the 2010 census, the region’s population is about 140,000 and the total area is about 320 square-kilometers.
In Isahaya City, the local government subsidizes the expense of seasonal influenza vaccination for all children who wish to receive vaccines from 6 months to 11 or 12 years of age (until graduation of elementary school). Subsidies were given from October 1st to February 28th in every season.
The vaccine
The vaccine used in this study contained 30 μg/ml of hemagglutinin for each of the recommended components as follows: A/California/7/2009(H1N1), A/Perth/16/2009(H3N2), and B/Brisbane/60/2008 in the 2011/12 season, and A/California/7/2009(H1N1), A/Victoria/361/2011(H3N2), and B/Wisconsin/01/2010 in the 2012/13 season. The influenza vaccine was given a dose of 0.25 ml per injection for children aged 6 month through 2 years of age, and a dose of 0.5 ml for children aged 3 through 12 years, following the Japanese guidelines. Children received influenza vaccine once or twice at medical facilities before the start of the influenza season. Children who received two vaccine doses were given 2-4 weeks apart according to the recommendation of the Ministry of Health, Welfare, and Labor in Japan.
Vaccinees were registered at the Health and Welfare Center of Isahaya City with their name and age data for the subsidy. Individual data were aggregated into the number of children in the vaccinated group (vaccinated at least once in a season) and unvaccinated group and stratified according to age. The information without individual records was sent to Niigata University for further analysis of vaccine effectiveness. The use of data without individual records for this study was approved by the Health and Welfare Center of Isahaya City and Isahaya Medical Association.
Case surveillance and definition
Isahaya Medical Association in Isahaya City, Nagasaki Prefecture has conducted an influenza registration program since the 2003/04 season (Kimura et al. 2011). The number of medical facilities that participated in this study was 80 in the 2011/12 season and 78 in the 2012/13 season. All 17 pediatric facilities and almost all 56 internal medicine outpatient medical facilities were enrolled. When patients visited the medical facilities with influenza-like-illness (ILI), such as having a sudden onset of fever (> 37.5°C) and sore throat, cough, or myalgia; clinicians used influenza rapid diagnostic test kits to screen for influenza A or B. Informed consent was obtained and the following information of the patients was collected: age, day of onset, diagnosis (influenza type A or B by rapid diagnosis test or clinically diagnosed), influenza vaccination status, and “most likely” route of transmission. In terms of the question for route of transmission, patient or patient’s parent chose a single answer where they thought they got infected with influenza, from a set of multiple choices; at home, at school/preschool, at the crowded place, or the other including unknown. Registration was commenced when the first ILI case occurred and ended in the end of April (week 18 of epidemic week) every season. This influenza case surveillance program was approved by the Ethical Committee of Niigata University Graduate School of Medical and Dental Sciences and Isahaya Medical Association. The overall study to evaluate influenza vaccine effectiveness is approved by the committee of Isahaya Medical Association.
Measurement of incidence rates of influenza and vaccine effectiveness
Overall incidence rate of influenza A or B infections in Isahaya City was calculated from the number of children who were positive with rapid test kits from the case surveillance program, divided by the population of children aged 1-11 years in each season. Age specific data, 1-2 years, 3-5 years, and 6-11 years, were also calculated by type and season. Vaccine effectiveness was estimated as [(1 − relative risk) × 100%] where the relative risk is the ratio of the incidence of vaccinated group over unvaccinated group. Incidence rate of vaccinated group was obtained by dividing the number of influenza cases in the respective type (A or B) diagnosed using the rapid diagnostic test kits who received influenza vaccination at least once, by the number of vaccinated children in 1-11 years from the record of the municipal registration program. On the other hand, incidence rate of unvaccinated group was obtained by dividing the number of unvaccinated influenza patients from the respective type registered to the case surveillance program, by the number of the unvaccinated population aged 1-11 years. The unvaccinated population was calculated by subtracting the number of vaccinated population in municipal registration record from the total population obtained by the city census. We computed vaccine effectiveness against influenza A and influenza B with all age and with subdivided age groups (1-2 years, 3-5 years, and 6-11 years). The chi-square test was used to compare the incidence rates between the vaccinated and unvaccinated groups. For all hypothesis tests, p value < 0.05 was considered statistically significant. Statistical analyses were conducted using R version 2.15.1 (R Foundation for Statistical Computing, Vienna, Austria).
Laboratory analysis
Virological confirmation of influenza A (H3N2 and H1N1pdm09) or B that circulated in the two seasons in Isahaya City was performed with selected samples collected at one of the pediatric clinics in the city. Throat swabs were obtained from patients positive with influenza rapid diagnostic test kits and kept in viral transport medium upon collection. The samples were sent to Niigata University for further laboratory analysis. Influenza virus isolation was performed by using Madin-Darby canine kidney (MDCK) cells. Typing and subtyping were done by Real-Time PCR as reported previously (Dapat et al. 2012).
Results
Vaccination coverage rate
The city census showed that the number of children from 1 to 11 years of age in Isahaya City was 14,562 and 14,282 on the first date of vaccination for seasonal influenza (October 1st) in 2011 and 2012, respectively.
According to the municipal vaccination record, 9,864 children were vaccinated and 4,698 were not vaccinated in 2011, and the numbers of the respective groups were 8,773 and 5,509 in 2012 (Table 1). Overall vaccination coverage rates in the two seasons were 67.7% and 61.4%. In the 2011/12 season, aggregated vaccination coverage rates were 75.3% in the 1-2 years of age group, 74.4% in the 3-5 years, and 62.2% in the 6-11 years. In the 2012/13 season, the figures for the respective groups were 66.4%, 66.0%, and 57.7%. The older children had a lower vaccination coverage rate in both seasons, and the vaccination coverage decreased in the second season.
Influenza epidemiology and the incidence rates
In Isahaya City, 2,412 children in 1-11 years of age were diagnosed as influenza A or B infections at the medical facilities between the week 52 of 2011 and the week 18 of 2012 in the first season, and 3,346 were diagnosed between the week 48 of 2012 and the week 18 of 2013 in the second season. In both seasons, co-circulation of influenza A and B was observed (Fig. 1).
The overall incidence rates of influenza A were 10.4% in the 2011/12 season, and was 16.8% in the 2012/13 season. The figures for influenza B were 5.9% and 6.6%, respectively (Table 2). For influenza A, the incidence rates were higher in the second season than the first season, but the rates over age groups (1-2 years, 3-5 years, and 6-11 years) did not show difference. For influenza B, the incidence rates were low in both two seasons, and the rates increased as children became older.
Vaccine effectiveness
The overall vaccine effectiveness against influenza A virus in children from 1-11 years of age in Isahaya City, was 23% (95% CI: 14%-31%) in the 2011/12 season and 13% (95% CI: 4%-20%) in the 2012/13 season, with statistical significance (Table 2). In the 2011/12 season, vaccine effectiveness against influenza A virus was apparent in 1-5 years [1-2 years: 55% (95% CI: 40%-66%); 3-5 years: 32% (95% CI: 17%-45%)]. However, there was no significance in those aged 6-11 years [8% (95% CI: −7%-21%)]. Similarly, in the 2012/13 season, vaccine effectiveness against influenza A virus was apparent in 1-5 years [1-2 years: 19% (95% CI: −1%-35%); 3-5 years: 20% (95% CI: 5%-33%)], but no significant effect was found in those aged 6-11 years [6% (95% CI: −5%-17%)].
The overall vaccine effectiveness in children against influenza B was 20% (95% CI: 8%-31%) in the 2011/12 with statistical significance. However, it was not significant in the 2012/13 season at 9% (95% CI: −4%-21%). In the 2011/12 season, the vaccine effectiveness against influenza B virus was apparent in 1-5 years [1-2 years: 40% (95% CI: −1%-65%); 3-5 years: 31% (95% CI: 2%-52%)], but there was no significance in 6-11 years [3% (95% CI: −14%-18%)]. In the 2012/13 season, vaccine effectiveness against type B was apparent in 3-5 years [29% (95% CI: 5%-47%)]. However, there was no significance for those under 2 years [−1% (95% CI: −82%-44%)] and more than 6 years [−5% (95% CI: −23%-10%)].
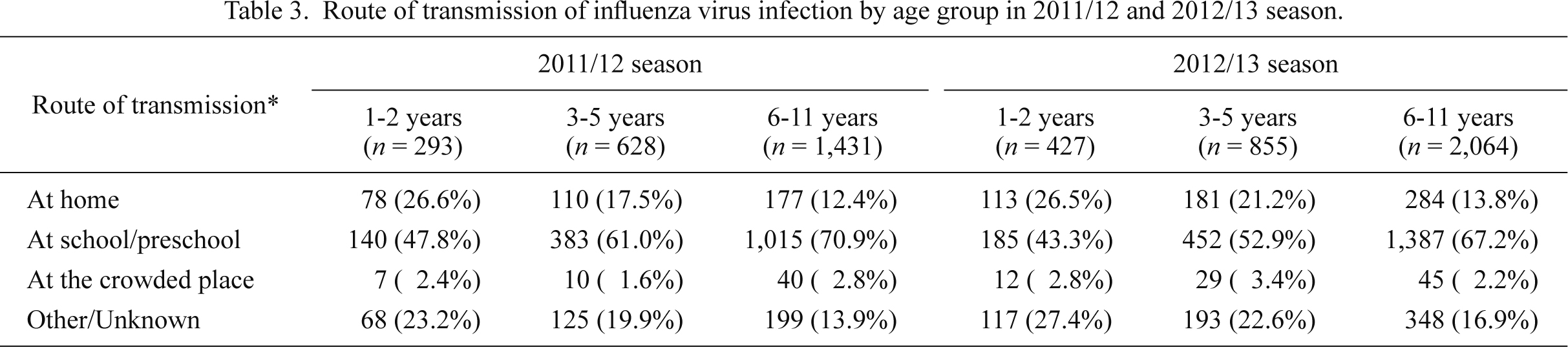
Route of transmission
Majority of patients (65.4% in the first season and 60.5% in the second season) replied that they thought they contracted the infection either at their school or preschool, and the rates increased with age (47.8% at 1-2 years, 61.0% at 3-5 years, and 70.9% at 6-11 years in the 2011/12 season, and 43.3%, 52.9% and 67.2%, respectively, in the 2012/13 season) (Table 3). In contrast, those who replied they contracted the infection at home reduced by age group (26.6% at 1-2 years, 17.5% at 3-5 years, 12.4% at 6-11 years in the 2011/12 season, and 26.5%, 21.2% and 13.8%, respectively, in the 2012/13 season). These results suggested that the school and the preschool are an important source of infection among children.
Virological confirmation of circulating strains
A total of 11 and 25 influenza isolates in the 2011/12 and the 2012/13 seasons, respectively, were obtained from influenza patients diagnosed using rapid test kits at the sentinel pediatric clinic in Isahaya City. Of the 11 isolates obtained in the 2011/12 season, 5 were influenza A (H3N2) and 6 were influenza B. Of the 6 of influenza B isolates, 4 (66.7%) belonged to the Victoria-lineage viruses, and the rest 2 (33.3%) were in Yamagata-lineage. Of the 25 isolates in the 2012/13 season, 18 were influenza A (H3N2) and 7 were influenza B. All influenza B isolates belonged to the Yamagata-lineage viruses. Influenza A (H1N1) pdm09 was not detected during either season.
Discussion
This study was conducted during two consecutive seasons in a community involving a total of 28,884 children-years in Japan. The trivalent influenza vaccine demonstrated a significant protective effect against both influenza A and B infections in the 2011/12 season and against influenza A infections in the 2012/13 season. Stratified by age, vaccine effectiveness was higher for younger children aged less than 6 years compared with those in children 6 years old and over, for both types of influenza infections during two seasons.
In Japan, some studies reported on influenza vaccine effectiveness but only a few found significant protective effects. A significant vaccine efficacy of 52% in influenza A and 59% in influenza B were found during 6 consecutive seasons diagnosed using rapid test kits among children less than 6 years in a community in Japan (Katayose et al. 2011). Other studies that estimated vaccine effectiveness among children in Japan during a single influenza season was to be 24%-44% (Fujieda et al. 2008; Ochiai et al. 2009; Yamaguchi et al. 2010). However, some vaccine effectiveness studies showed no statistical significance (Ohkuma et al. 2002; Maeda et al. 2004). One of the strengths of this study is that our investigation had larger sample size (28,884 children-years) than other vaccine effectiveness studies in Japan (346-14,788 children-years) (Ohkuma et al. 2002; Maeda et al. 2004; Fujieda et al. 2008; Ochiai et al. 2009; Yamaguchi et al. 2010; Katayose et al. 2011). To evaluate vaccine effectiveness, the community cohort that involved the entire local city area like this study is essential.
Although the vaccine dose has been doubled, our study demonstrated lower vaccine effectiveness, compared to the previous reports. There are several reasons for lower vaccine effectiveness. There was a mismatch between the circulating influenza strains and the vaccine strain in the 2011/12 season in Japan. According to the antigenic analysis of influenza A (H3N2) circulated in Japan in 2011/12 season, 34% had an eight-fold or greater reduction in hemagglutination-inhibition titer compared with the vaccine virus (Infectious Disease Surveillance Center, National Institute of Infectious Diseases 2012). Of all the Influenza B strains collected in Japan, two-thirds belonged to the Victoria lineage, contained the trivalent influenza vaccine in the 2011/12 season, whereas the remainder belonged to the Yamagata lineage (Infectious Disease Surveillance Center, National Institute of Infectious Diseases 2012). The proportion of the Victoria lineage and the Yamagata lineage was almost same in Isahaya City. Thus, for both subtypes A (H3N2) and B, antigenic differences may have existed between the circulating and vaccine strains in 2011/12 season, and this may be the reason for lower vaccine effectiveness.
For the 2012/13 season, the decreased effectiveness of A (H3N2) may be explained by a mismatch between egg-derived vaccine strain and circulating strains (Infectious Disease Surveillance Center, National Institute of Infectious Diseases 2013, WHO Collaborating Centre for Reference and Research on Influenza, National Institute for Medical Research 2013). According to the reports, 94% of circulated A(H3N2) showed 8-16 times reductions of hemagglutination-inhibition titer compared to egg-derived vaccine strain, A/Victoria/361/2011. In contrast, the MDCK isolated vaccine strain (A/Victoria/361/2011) matched with the circulating strains. This can be the reason for the lower vaccine effectiveness for influenza A in the second season due to antigenic mismatch. For influenza B, there was no antigenic difference in the 2012/13 season, having both vaccine and circulating strains to be in Yamagata lineage. However, the vaccine effectiveness was lower than the 2011/12 season. It is conceivable that children who were infected in the previous season have already obtained an antibody against the strains in the following season of the same antigenicity. The certain immune level by infections, which surpass the responses induced by vaccination, was already obtained in both vaccinated and unvaccinated groups. As a result, it led to lower vaccine effectiveness in the season because of a smaller difference between vaccinated and unvaccinated groups in terms of proportions of subjects who had protective antibody levels.
For influenza B, the vaccine effectiveness was generally lower than influenza A for both seasons. In some of the previous Japanese reports, vaccine effectiveness for influenza B was lower than influenza A (Sugaya et al. 1994) or did not show statistical significance even though the vaccine strain and the circulating strains matched (Yamaguchi et al. 2010). The comprehensive reasons remained unknown, but one of the explanations from our study is that the incidence rate was always higher in the school-age children compared to the younger children with influenza B for the two seasons. Age stratified vaccine effectiveness showed that the protection is smaller in the school-age children compared to the younger children. It may be the reason for the diminished vaccine effectiveness for influenza B.
Age-stratified estimation showed that vaccine effectiveness was superior in younger children over both seasons and for both virus types. In general, young children with no detectable antibody to influenza are less likely to develop immunologic response to the influenza vaccine than are older children with a preexisting antibody (Hurwitz et al. 2000; Jefferson et al. 2012). However in this study, children less than 6 years of age had benefited from improved effectiveness compared with school-age children. One of the reasons for children less than 6 years showed significant vaccine effectiveness is that younger children have a higher vaccination coverage rate. High vaccination coverage rate reduces the risk of infection due to herd effect (Van Vlaenderen et al. 2013). In addition, children aged less than 6 years could have less chance of infection than school-age children. It is presumed that school-age children have a wider range of activities and a higher frequency of contact with other children at school, compared with younger children who are likely to stay at home with less contact often limited to their guardians. According to the question for the route of transmission, as children grow older, the opportunity for contracting infection at their school increased. School-age children spend more time with other children at school (Vazquez-Prokopec et al. 2013). As they contact with others more frequently at school, they would be exposed more to virus and have more chance of infection for both vaccinated and unvaccinated groups. Thus, the vaccine effectiveness in school-age children may be diminished. The increase of vaccination dose for children is a probable cause of significant vaccine effectiveness for children less than 6 years, although we were not able to estimate the effectiveness before the change of vaccination dose.
Apart from vaccine effectiveness, the vaccination coverage gradually decreased with advancing age. Cost of subsidy for children in Isahaya City was the same from infants until the graduation of elementary school. In a previous study conducted among children, the vaccination coverage rate was 41.6% for children aged from 6-23 months and 26.1% for those aged 2-8 years (Ritzwoller et al. 2005). One of the reasons for the lower vaccination rate in older children was that their parents tended to visit the pediatric facilities less frequently and get less information about influenza vaccination (Ando 2012). Furthermore, it was reported that as the children get older, some parents believe that their children are already immune to influenza infections (Toyoshima 2012). For these reasons, higher age children withhold of vaccination and the vaccination coverage decreased with age.
This study has several limitations. Firstly, the study was not double-blind or randomized by design and choice of vaccinated or non-vaccinated was self-selected by parents. Thus, some bias could be introduced if the behavior of seeking medical care was different between vaccinated and unvaccinated. It is likely that those who did not go to vaccination tend not to consult with medical facilities even though they developed ILI. This may lead to underestimation of the vaccine effectiveness. Secondly, vaccination registration included only information of vaccinees. Thus the number of unvaccinated was calculated by the differences in number of vaccinees and the city census population. No authentic figures of unvaccinated group could be obtained. Thirdly, missed influenza cases in the community may have existed. Although all pediatric facilities in the city participated in the registration program, a certain number of children may have not consulted to the facilities due to low motivation for medical seeking, or they consulted to the facilities for medical care in other towns adjacent to the Isahaya City. Fourthly, bias may occur while estimating vaccine effectiveness with case findings using the rapid diagnosis test kits due to the relative lack of sensitivity and specificity compared with those using molecular techniques (Orenstein et al. 2007). If the number of patients with ILI was large in the season, more children were likely to have been misclassified, which have caused the bias in the estimation of vaccine effectiveness (Hirota et al. 2008). Because we could not obtain the data on the number of children who were vaccinated in Isahaya city before the increase of vaccine dose for children, we were not able to estimate the effectiveness before the change. In addition, in this study, it was unknown whether children less than 6 years attended preschools or day cares. The vaccine effectiveness might be variable, depending on the attendance of day cares or staying at home in terms of the chance of contact, although we could not obtain the relevant information.
In conclusion, a significant protective effect was found for children 1-11 years of age with trivalent influenza vaccine over two consecutive seasons. Of note, vaccine effectiveness was higher in influenza A compared to influenza B and protection was higher in infants than older children. Our community study encourages vaccination prior to influenza seasons to protect children from influenza infections. We plan to continue this study in the same cohort and to investigate other areas in Japan to monitor the effectiveness of the vaccination.
Acknowledgements
This study was supported by the Grants-in-Aid for emerging and re-emerging infectious disease, the Ministry of Health, Labour and Welfare of Japan (H23-Emerging infectious disease-003). We thank the clinicians in Isahaya City for participating in an influenza registration program. We thank the Isahaya Medical Association and the Health and Welfare Center of Isahaya City for providing data.
Conflict of Interest
The authors declare no conflict of interest.
References
-
Ando,
Y.
(2012) The questionnaire survey for guardians about Haemophilus influenza type b conjugated vaccine and 7-valent pneumococcal conjugated vaccine. J. Natl. Inst. Public Health, 61, 492-493 (in Japanese).
-
Centers for Disease Control and Prevention
(2012) Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices — United States, 2012-13 influenza season. MMWR Morb. Mortal. Wkly. Rep., 61, 613-618.
-
Clemens,
J.,
Brenner,
R.,
Rao,
M.,
Tafari,
N. &
Lowe,
C.
(1996) Evaluating new vaccines for developing countries. Efficacy or effectiveness? JAMA, 275, 390-397.
-
Clemens,
J.D. &
Shapiro,
E.D.
(1984) Resolving the pneumo-coccal vaccine controversy: are there alternatives to randomized clinical trials? Rev. Infect. Dis., 6, 589-600.
-
Dapat,
I.C.,
Dapat,
C.,
Baranovich,
T.,
Suzuki,
Y.,
Kondo,
H.,
Shobugawa,
Y.,
Saito,
R. &
Suzuki,
H.;
Japanese Influenza Collaborative Study Group
(2012) Genetic characterization of human influenza viruses in the pandemic (2009-2010) and post-pandemic (2010-2011) periods in Japan. PLoS One, 7, e36455.
-
Fujieda,
M.,
Maeda,
A.,
Kondo,
K.,
Fukushima,
W.,
Ohfuji,
S.,
Kaji,
M. &
Hirota,
Y.
(2008) Influenza vaccine effectiveness and confounding factors among young children. Vaccine, 26, 6481-6485.
-
Health Service Bureau, Ministry of Health, Labour and Welfare
(2011) For stable supply measures of influenza vaccine. [Cited: August 8, 2011] http://www.jshp.or.jp/cont/11/0812-2.pdf [Accessed: January 15, 2014] (in Japanese).
-
Hirota,
Y.,
Fukushima,
W.,
Fujieda,
M.,
Ohfuji,
S. &
Maeda,
A.
(2008) Essential tools for assessing influenza vaccine efficacy in improperly conducted studies: a Japanese perspective. Vaccine, 26, 6455-6458.
-
Hurwitz,
E.S.,
Haber,
M.,
Chang,
A.,
Shope,
T.,
Teo,
S.T.,
Giesick,
J.S.,
Ginsberg,
M.M. &
Cox,
N.J.
(2000) Studies of the 1996-1997 inactivated influenza vaccine among children attending day care: immunologic response, protection against infection, and clinical effectiveness. J. Infect. Dis., 182, 1218-1221.
-
Infectious Disease Surveillance Center, National Institute of Infectious Diseases
(2012) Analysis of influenza virus isolates in 2011-12 season. Infectious Agents Surveillance Report, 33, 288-294 (in Japanese).
-
Infectious Disease Surveillance Center, National Institute of Infectious Diseases
(2013) Analysis of influenza virus isolates in 2012-13 season. Infectious Agents Surveillance Report, 34, 328-334 (in Japanese).
-
Jefferson,
T.,
Rivetti,
A.,
Di Pietrantonj,
C.,
Demicheli,
V. &
Ferroni,
E.
(2012) Vaccines for preventing influenza in healthy children. Cochrane Database Syst. Rev., 8, CD004879.
-
Katayose,
M.,
Hosoya,
M.,
Haneda,
T.,
Yamaguchi,
H.,
Kawasaki,
Y.,
Sato,
M. &
Wright,
P.F.
(2011) The effectiveness of trivalent inactivated influenza vaccine in children over six consecutive influenza seasons. Vaccine, 29, 1844-1849.
-
Kimura,
Y.,
Saito,
R.,
Tsujimoto,
Y.,
Ono,
Y.,
Nakaya,
T.,
Shobugawa,
Y.,
Sasaki,
A.,
Oguma,
T. &
Suzuki,
H.
(2011) Geodemographics profiling of influenza A and B virus infections in community neighborhoods in Japan. BMC Infect. Dis., 11, 36.
-
Kubo,
N.,
Ikematsu,
H.,
Nabeshima,
S.,
Yamaji,
K.,
Nabeshima,
A.,
Kondou,
H.,
Chong,
Y.,
Kashiwagi,
S. &
Hayashi,
J.
(2003) Evaluation of an immunochromatography test kit for rapid diagnosis of influenza. The Journal of the Japanese Association for Infectious Diseases, 77, 1007-1014 (in Japanese).
-
Maeda,
T.,
Shintani,
Y.,
Nakano,
K.,
Terashima,
K. &
Yamada,
Y.
(2004) Failure of inactivated influenza A vaccine to protect healthy children aged 6-24 months. Pediatr. Int., 46, 122-125.
-
Mitamura,
K.,
Yamazaki,
M.,
Ichikawa,
M.,
Kimura,
K.,
Kawakami,
C.,
Shimizu,
H.,
Watanabe,
S.,
Imai,
M.,
Shinjo,
M.,
Takeuchi,
Y. &
Sugaya,
N.
(2004) Evaluation of an immunochromatography test using enzyme immunoassay for rapid detection of influenza A and B viruses. The Journal of the Japanese Association for Infectious Diseases, 78, 597-603 (in Japanese).
-
Ochiai,
H.,
Fujieda,
M.,
Ohfuji,
S.,
Fukushima,
W.,
Kondo,
K.,
Maeda,
A.,
Nakano,
T.,
Kamiya,
H. &
Hirota,
Y.;
Influenza Vaccine Epidemiology Study Group
(2009) Inactivated influenza vaccine effectiveness against influenza-like illness among young children in Japan: with special reference to minimizing outcome misclassification. Vaccine, 27, 7031-7035.
-
Ohkuma,
K.,
Teramoto,
Y.,
Fukuta,
M.,
Takahashi,
H.,
Yano,
T.,
Sugiyama,
A.,
Nakayama,
O. &
Kamiya,
H.
(2002) Efficacy and safety of influenza vaccine for infants in Mie Prefecture in 2000/2001 prevalent season. Annual Report of Mie Prefecture Health and Environment Research Institute, 4, 86-93 (in Japanese).
-
Orenstein,
E.W.,
De Serres,
G.,
Haber,
M.J.,
Shay,
D.K.,
Bridges,
C.B.,
Gargiullo,
P. &
Orenstein,
W.A.
(2007) Methodologic issues regarding the use of three observational study designs to assess influenza vaccine effectiveness. Int. J. Epidemiol., 36, 623-631.
-
Osterholm,
M.T.,
Kelley,
N.S.,
Sommer,
A. &
Belongia,
E.A.
(2012) Efficacy and effectiveness of influenza vaccines: a systematic review and meta-analysis. Lancet Infect. Dis., 12, 36-44.
-
Palache,
A.
(2011) Seasonal influenza vaccine provision in 157 countries (2004-2009) and the potential influence of national public health policies. Vaccine, 29, 9459-9466.
-
Ritzwoller,
D.P.,
Bridges,
C.B.,
Shetterly,
S.,
Yamasaki,
K.,
Kolczak,
M. &
France,
E.K.
(2005) Effectiveness of the 2003-2004 influenza vaccine among children 6 months to 8 years of age, with 1 vs 2 doses. Pediatrics, 116, 153-159.
-
Sugaya,
N.,
Nerome,
K.,
Ishida,
M.,
Matsumoto,
M.,
Mitamura,
K. &
Nirasawa,
M.
(1994) Efficacy of inactivated vaccine in preventing antigenically drifted influenza type A and well-matched type B. JAMA, 272, 1122-1126.
-
Toyoshima,
Y.
(2012) How the novel influenza (pandemic 2009) can influence the attitudes of elementary and junior high school students toward vaccination. Jpn. J. Public Health, 59, 390-398 (in Japanese).
-
Van Vlaenderen,
I.,
Van Bellinghen,
L.A.,
Meier,
G. &
Nautrup,
B.P.
(2013) An approximation of herd effect due to vaccinating children against seasonal influenza: a potential solution to the incorporation of indirect effects into static models. BMC Infect. Dis., 13, 25.
-
Vazquez-Prokopec,
G.M.,
Bisanzio,
D.,
Stoddard,
S.T.,
Paz-Soldan,
V.,
Morrison,
A.C.,
Elder,
J.P.,
Ramirez-Paredes,
J.,
Halsey,
E.S.,
Kochel,
T.J.,
Scott,
T.W. &
Kitron,
U.
(2013) Using GPS technology to quantify human mobility, dynamic contacts and infectious disease dynamics in a resource-poor urban environment. PLoS One, 8, e58802.
-
Weinberg,
G.A. &
Szilagyi,
P.G.
(2010) Vaccine epidemiology: efficacy, effectiveness, and the translational research roadmap. J. Infect. Dis., 201, 1607-1610.
-
WHO Collaborating Centre for Reference and Research on Influenza, National Institute for Medical Research
(2013) September 2013 interim report. http://www.nimr.mrc.ac.uk/documents/about/NIMR-report-Sep2013final.pdf [Accessed: January 15, 2014].
-
World Health Organization
(2009) Influenza fact sheet No. 211. April 2009. http://www.who.int/mediacentre/factsheets/fs211/en/index.html. [Accessed: October 31, 2013].
-
Yamaguchi,
S.,
Ohfuji,
S. &
Hirota,
Y.
(2010) Influenza vaccine effectiveness in primary school children in Japan: a prospective cohort study using rapid diagnostic test results. J. Infect. Chemother., 16, 407-413.