Abstract
Low or high counts of white blood cells (WBCs) and WBC subtypes can be a predictor of morbidity and mortality in several clinical settings. However, the correlations of WBC and its subtypes with acute kidney injury (AKI) and mortality remain unresolved in critically ill patients. The counts of WBC and subtypes, such as neutrophil, lymphocyte, monocyte, and eosinophil, were measured in 2,079 patients admitted to the intensive care unit (ICU) from June 2004 through June 2010. The non-linear relationship between WBC counts and AKI risk was initially explored by a restricted cubic spline analysis. The odds ratios (ORs) for AKI and 1-year mortality were calculated after adjustment for multiple covariates. The relationship between WBC counts and AKI risk was U-shaped. Accordingly, we divided patients into quintiles according to the counts of WBC or subtypes. The 1st and 5th quintiles of WBC counts had greater ORs for AKI (1.42 and 2.05, respectively) and mortality (1.40 and 1.36, respectively) compared with the 3rd quintile. After stratification by WBC subtype, the 5th quintile of neutrophil counts and the 1st quintiles of lymphocyte and monocyte counts tended to have higher ORs for AKI (1.69, 1.40, and 1.77, respectively). For mortality, the 1st quintiles of neutrophil, lymphocyte, and eosinophil counts were associated with higher mortality compared with the 3rd quintile (the ORs were 1.48, 1.57, and 1.42, respectively). Both leukopenia and leukocytosis are associated with AKI and mortality risk in critically ill patients. This result may be attributable to the change in the subtype counts.
Introduction
Acute kidney injury (AKI) is a major health concern because it is associated with substantial morbidity and mortality (Chertow et al. 2005; Ishani et al. 2009). However, despite improvements in therapy, AKI remains highly prevalent, especially in critically ill patients in the intensive care unit (ICU) (Clermont et al. 2002). AKI in the ICU has a high mortality rate that reaches 80% (Turney 1996). This rate has remained relatively unchanged despite improved therapies (Ympa et al. 2005). For these reasons, it is clinicians’ essential responsibility to monitor and diminish the factors contributing to AKI.
Each white blood cell (WBC) subtype, including neutrophils, lymphocytes, monocytes, and eosinophils, has a discrete role in inflammation, host defense, and repair. Previously, counts of WBC and its subtypes have been documented to be a predictor of morbidity and mortality in several clinical settings (Knaus et al. 1985; Felmet et al. 2005; Abidi et al. 2011; Merino et al. 2012). These components are one of the immunologic factors that play a pivotal role in most processes of organ damage (Deitch 1992). This role can apply to the issue of kidney damage. It is known that alteration of WBC counts is associated with the risk of long-term kidney outcomes, such as chronic kidney disease and end-stage renal disease (Erlinger et al. 2003; Keane et al. 2003; Fried et al. 2004). The risk of AKI may also be graded based on the WBC count (Bagshaw et al. 2008). However, each role of WBC and WBC subtypes in AKI is not fully established in a clinical setting, although major advances are ongoing in the experimental research (Burne et al. 2001; Praga and Gonzalez 2010). Above all, it is unknown whether counts of WBC and its subtypes predict AKI in high-risk patients who are admitted to the ICU. Testing for WBC and subtype counts has the advantages of simplicity, inexpensiveness, and wide availability. These aspects lead to clinical significance that may be comparable with that of other immunologic markers, such as cytokines and chemokines. Furthermore, the explanation of the correlation between AKI and other immunologic markers will be limited before correlations with WBC and its subtypes are established. Herein, we aimed to verify the predictive value of WBC and WBC subtype counts on AKI and mortality in a large cohort of ICU patients.
Methods
Patients and data collection
The institutional review board of Seoul National University Bundang Hospital approved the study (no. B-1304/200-109). A total of 2,823 patients were admitted from June 2004 through June 2010 to the ICU at Seoul National University Bundang Hospital, Gyeonggi-do, Korea. The patients were followed up until June 30, 2013. We excluded patients younger than 18 years old (n = 49) and patients previously diagnosed with end-stage renal disease who were on dialysis (n = 94). Patients for whom data, such as serum creatinine levels or urine output, were missing were also excluded (n = 9). Lastly, patients with hematologic malignancy were excluded (n = 64). If patients were admitted more than once to the ICU, only the first admission was counted as a single case. Consequently, 2,079 cases were reviewed retrospectively using electronic medical records.
Clinical parameters, such as age, sex, weight, systolic/diastolic blood pressure, primary diagnosis, underlying chronic kidney disease, history of malignancy, the need for mechanical ventilation, and the use of vasoactive drugs, were recorded. Chronic kidney disease was diagnosed as either kidney damage or glomerular filtration rate of less than 60 ml/min/1.73 m2 for 3 months before the admission to the ICU. The primary diagnosis was categorized as cardiovascular disease, sepsis, surgical admission, and others. The Acute Physiology and Chronic Health Evaluation (APACHE) ІІ score was used to assess illness severity (Knaus et al. 1985). Changes in serum creatinine levels and urine output were measured after ICU admission, and the urine output data were recorded hourly. The counts of WBCs and WBC subtypes, such as neutrophils, lymphocytes, monocytes, and eosinophils, were measured using an automated hematology analyzer (XE-2100, Sysmex, Kobe, Japan). There were no missing data for any of the variables.
The risk of AKI was determined from admission to 15 days in the ICU. For the definition and staging of AKI, both the serum creatinine concentration and the urine output criteria that were used adhered to the guidelines proposed by Kidney Disease: Improving Global Outcomes (KDIGO) ((KDIGO) acute kidney injury work group 2012). The 1-year mortality from all causes was considered as the primary outcome. Mortality data were obtained from the national database of Statistics Korea.
Statistical analysis
All of the analyses and calculations were performed using STATA (STATA version 12.0, StataCorp LP, College Station, Texas, USA). The data are presented as the means ± standard deviation (s.d.) for continuous variables and as proportions for categorical variables. Based on variable distributions using histograms, the variables with a non-normal distribution are expressed as the median [interquartile range (IQR)]. The chi-squared test was used to compare the categorical variables. Comparisons between normally distributed continuous variables were made using an analysis of variance (ANOVA) or a post-hoc analysis of least significant difference (LSD) according to the number of comparison groups. Comparisons between non-normally distributed continuous variables were measured by either the Kruskal-Wallis test or the Mann-Whitney U test, depending on the number of comparison groups. The dose-response relationship (in particular, the non-linear relationship) between WBC counts and AKI risk was initially explored by a restricted cubic spline analysis (Desquilbet and Mariotti 2010), and there was a U-shaped relationship between these variables. Due to the non-linear relationship, we divided the patients into quintile groups according to the counts of WBC or its subtypes. Next, the odds ratios (ORs) and 95% confidence intervals (CIs) for AKI and 1-year mortality were calculated using the logistic regression model after stepwise adjustment for multiple confounders. A P value of less than 0.05 was considered significant.
Results
Baseline characteristics
The baseline characteristics of patients are shown and compared among the quintiles of WBC counts (Table 1). For the 2,079 subjects, the mean age was 68 years old. All of the subjects were of Asian descent. Most of the patients were admitted to the ICU because of medical problems (n = 2,033) rather than surgical problems (n = 46). More specifically, 649 patients (31.2%) were admitted to the ICU because of cardiovascular disease. Sepsis was the cause of admission for 95 patients (4.6%). The median counts of WBC and its subtypes were as follows: WBCs, 11,350/μL; neutrophils, 9,229/μL; lymphocytes, 1,025/μL; monocytes, 400/μL; and eosinophils, 61/μL. The median length of stay in the hospital was 22 days (IQR, 11 to 45 days). The study subjects were followed for a median duration of 543 days (IQR, 39 to 1,762 days).
The 5th quintile patients were more likely to be younger, overweight, and septic; to receive a vasoactive drug; and to have a high serum creatinine level or a high APACHE ІІ score compared with the 3rd quintile. The 1st quintile patients had a greater tendency to be female, septic, and hypotensive; to have a history of malignancy; and to receive mechanical ventilation compared with the 3rd quintile.
Risk of AKI according to WBC and WBC subtype counts
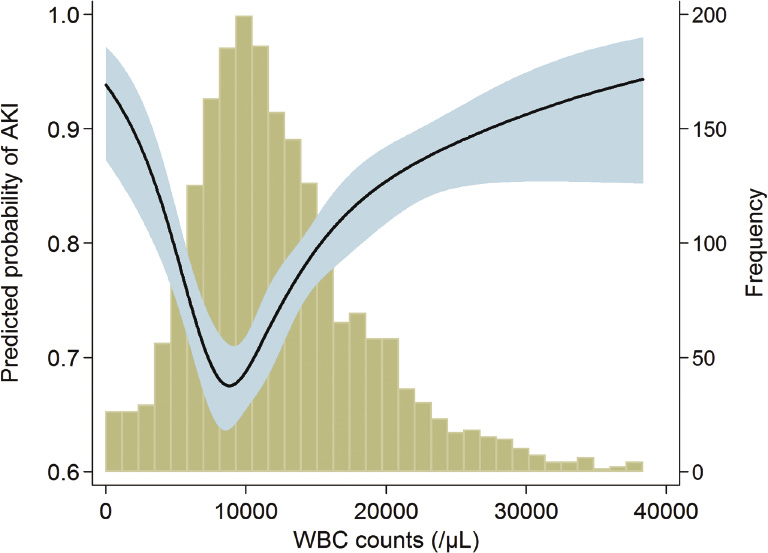
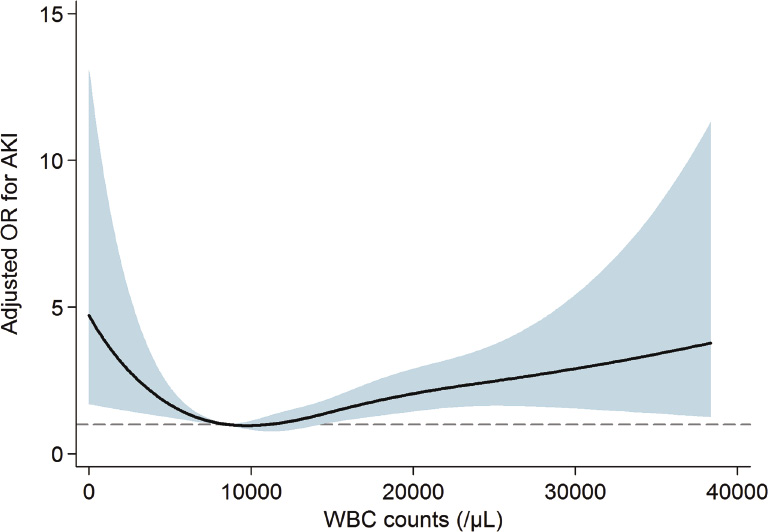
A total of 76.8% of the subjects had AKI within 15 days after admission to the ICU. The proportion in each AKI stage among the AKI cases was as follows: stage 1, 47.6%; stage 2, 28.5%; and stage 3, 23.9%. Among the patients with stage 3 AKI, 157 subjects (41.1%) received renal replacement therapy. Fig. 1 shows the relationship between WBC counts and the predicted probability of AKI. The relationship between WBC counts and AKI risk appeared to be U-shaped. When adjustments for multiple covariates were performed, the relationship between WBC counts and ORs for AKI was also U-shaped (Fig. 2). Based on the above observations, we divided patients into quintile groups according to WBC counts and assigned a reference to the 3rd quintile throughout all analyses. Table 2 shows the risk of AKI according to the quintile groups of WBC counts. In a univariate analysis, the 5th quintile group had a greater OR for AKI than did the 3rd quintile. The 1st quintile group had a higher tendency toward AKI than did the 3rd quintile, although the statistical significance was not evident. However, the AKI risk of the 1st quintile group was significantly greater than that of the 2nd quintile: OR (95% CI), 1.41 (1.035 to 1.924); P = 0.030. After adjusting for multiple variables, the 1st and the 5th quintiles had greater ORs for AKI compared with the 3rd quintile (all Ps < 0.05).
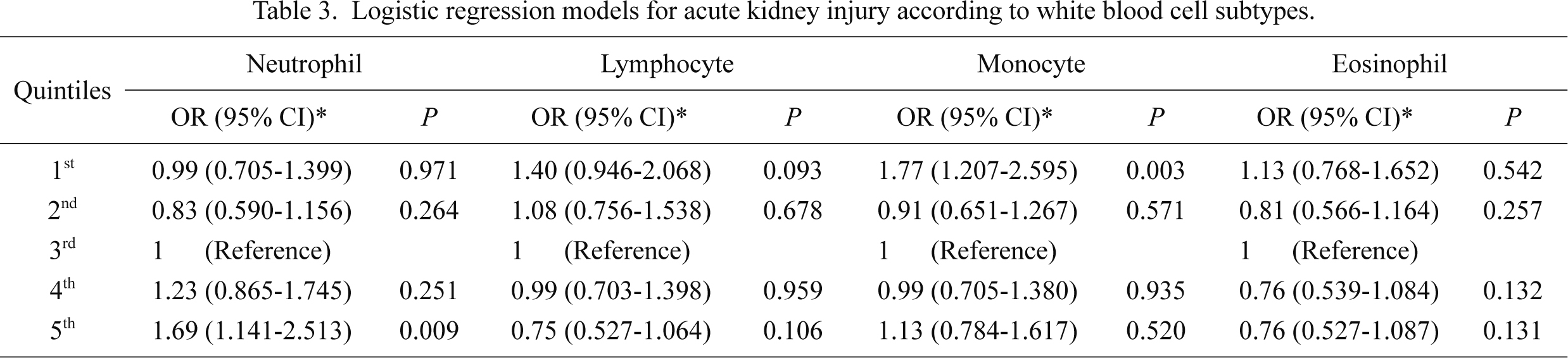
The risk of AKI was evaluated after stratification by WBC subtype (Table 3), in which further adjustment for the counts of subtypes other than the analyzed subtype was performed. The relationship with AKI was different according to subtype, as follows. Neutrophilia, but not neutropenia, had a tendency toward AKI risk, whereas a reverse trend was shown for lymphocytes and monocytes, although the relationship with lymphocytes was not statistically significant. Moreover, eosinophil counts did not correlate with the risk of AKI.
Risk of mortality according to WBC and WBC subtype counts
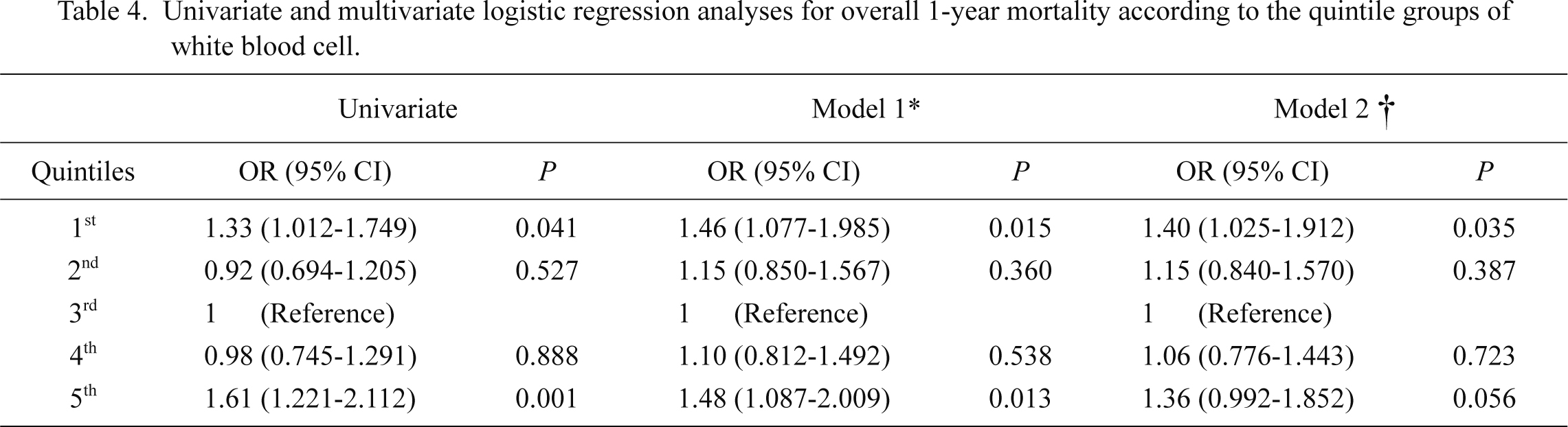
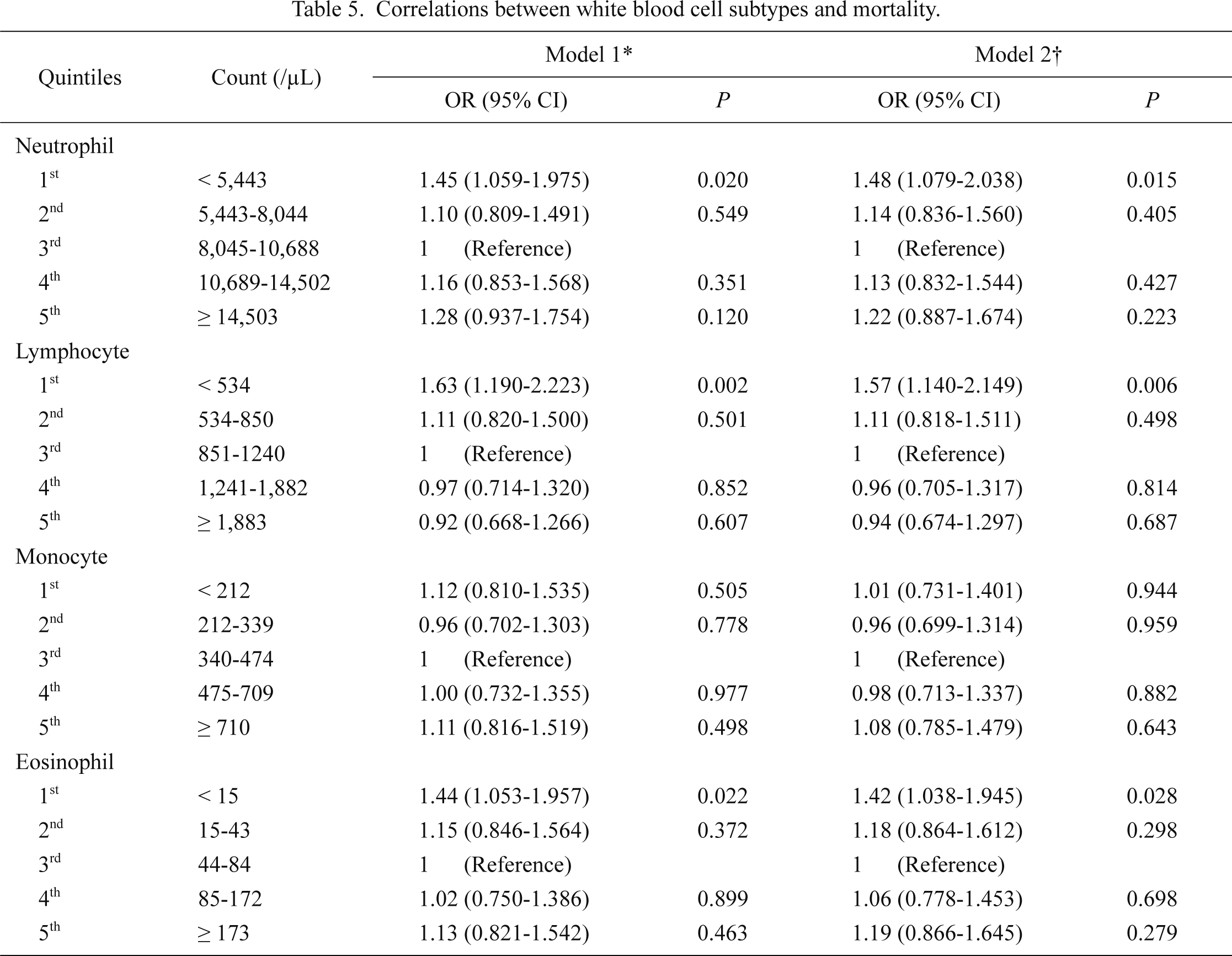
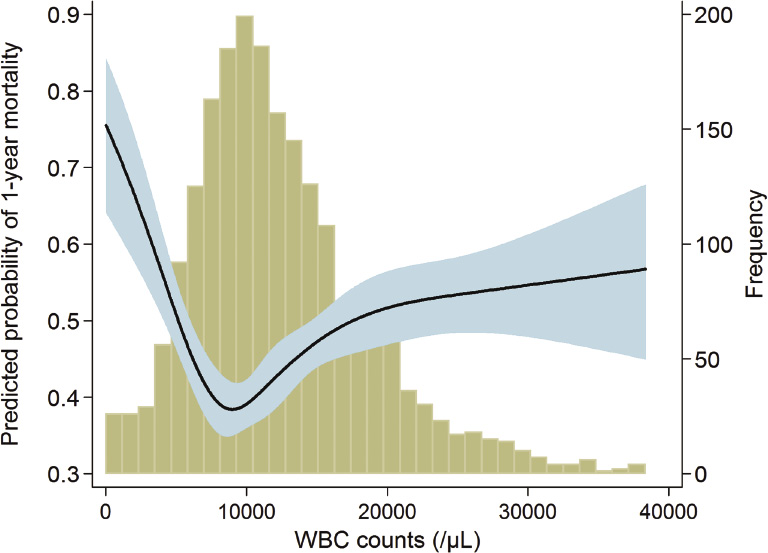
Throughout the follow-up period, 1,367 (65.8%) of all ICU patients died, and the mortality rate was 67.4 deaths per 100,000 patient-days. As shown in Fig. 3, the correlation between WBC counts and mortality seemed to be U-shaped. After adjustment for multiple confounders, both leukopenia and leukocytosis seemed to have greater ORs than did the mid-portion of the WBC range (Fig. 4). Accordingly, we divided patients equally into quintiles to perform the above analyses of AKI. Table 4 shows the risk of 1-year mortality according to the WBC quintiles. In a univariate analysis, the 1st and 5th quintiles had an association with a high risk of mortality. Most confounders did not hamper this correlation, although the impact of AKI was relatively strong enough to decrease the correlation’s significance. We further evaluated the correlation with mortality risk after stratification by WBC subtype (Table 5). The correlation with mortality risk for neutrophil counts was different from the correlation with AKI; neutropenia, but not neutrophilia, had a tendency toward mortality risk. For lymphocyte counts, the lowest quintile had a greater OR for mortality compared with the 3rd quintile, and the statistical significance was evident. Monocyte counts did not have a relationship with mortality risk. However, eosinophil counts exhibited a significant trend, with eosinopenia predicting high mortality risk. For all of the above correlations between WBC subtypes and mortality risk, the presence of AKI did not decrease the correlation significances.
Discussion
Mortality among ICU patients is extremely high, and AKI worsens this outcome. To predict and manage the mortality attributable to AKI, several markers have been considered. Among these markers, counts of WBC and its subtypes are easily accessible and widely examined, but correlations with AKI and mortality have not been extensively studied in the ICU setting. The present study first examined the above issue in ICU patients. There was a U-shaped relationship between WBC count and AKI risk. Based on the results of WBC subtype counts, high AKI risk in leukopenia may be attributable to both lymphopenia and monocytopenia, whereas high AKI risk in leukocytosis may be due to neutrophilia. There was also a U-shaped relationship between WBC count and mortality risk. However, the pattern of the correlation between WBC subtypes and mortality was not the same as for the above AKI results; neutropenia and lymphopenia tended to result in a greater risk of mortality, but monocyte counts did not correlate with mortality. Instead, eosinopenia was a significant predictor of high mortality, in contrast to the finding that the eosinophil count was not correlated with AKI risk.
The role of WBC and its subtypes in the mechanism of AKI has been extensively investigated in experimental studies, whereas clinical studies are relatively insufficient. It is well known that both leukocytosis and leukopenia are associated with mortality risk (Knaus et al. 1985), which results in a U-shaped relationship between WBC count and mortality risk. However, whether this relationship can be applied to the field of AKI is unknown. Previous clinical studies have consistently demonstrated that leukocytosis increases the risk of morbidities such as cardiovascular disease, metabolic syndrome, and diabetes mellitus (Madjid et al. 2004; Gkrania-Klotsas et al. 2010; Babio et al. 2013). The present study builds on previous results because the correlation between WBC count and AKI is also prominent as other diseases. Furthermore, the present study confirms that there is a U-shaped relationship with AKI, which means that both leukocytosis and leukopenia increase the risk of AKI. Stratified analyses of WBC subtypes showed that neutrophilia, lymphopenia, and monocytopenia were associated with AKI risk. Although the underlying mechanism cannot be determined from this observational study, the correlation pattern of each WBC subtype may suggest the following: the correlation with AKI in leukocytosis is attributable to neutrophilia, whereas the correlation in leukopenia is attributable to both lymphopenia and monocytopenia.
Neutrophils generate reactive oxygen species and thus can lead to the destruction of normal self cells in inflamed tissue. Several experimental models, such as ischemic, nephrotoxic, and endotoxemia-induced AKI models, are associated with an increase in infiltrating neutrophils in the kidney (Cunningham et al. 2002; Melnikov et al. 2002; Faubel et al. 2007). It has been revealed that T and B lymphocytes have a role in modulating the innate and adaptive inflammatory responses, which may lead to the inflammatory process of AKI (Burne-Taney et al. 2003; Day et al. 2006). However, other subtypes of lymphocytes, such as regulatory T lymphocytes, have strong immunosuppressive properties, and a depletion of this lymphocyte worsens kidney damage (Kinsey et al. 2009). Additionally, natural killer T cells, a unique subtype of lymphocyte, may have both pro-inflammatory and protective roles in AKI (Li et al. 2007; Yang et al. 2011). Monocytes are recruited to the kidney and differentiate into macrophages or dendritic cells during the AKI event. There, the cells also have both pro-inflammatory and healing effects in the kidney (Lee et al. 2011). Although lymphocytes and monocytes have competing roles in AKI, the present study results can be explained by experimental findings, as follows: neutrophilia leads to AKI by pro-inflammatory function, whereas lymphopenia and monocytopenia result in AKI due to a lack of protective function. Further clinical and experimental studies are needed to address this hypothesis.
The U-shaped relationship observed between WBC counts and mortality in the ICU setting is consistent with previous findings (Knaus et al. 1985). We additionally evaluated the relationship of each WBC subtype with mortality, which was not the same as the AKI results. The effects of neutropenia, lymphopenia, and eosinopenia on mortality support recent findings, although the study settings are not exactly same (Knaus et al. 1985; Felmet et al. 2005; Kuderer et al. 2006; Ducloux et al. 2010; Abidi et al. 2011; Merino et al. 2012). Based on the observations of the study, the correlation with mortality in leukopenia may be attributable to neutropenia and lymphopenia, whereas the correlation in leukocytosis may be attributable to the composite outcome of all WBC subtypes. The presence of AKI relatively decreased the significance of the correlation of mortality with WBC quintiles, but a definite conclusion on this topic cannot be drawn from the present study alone. It is plausible that the impact of WBC or its subtypes on mortality is both dependent on and independent of AKI because several morbidities other than AKI are known to be associated with WBC and its subtypes (Madjid et al. 2004; Felmet et al. 2005; Dragu et al. 2008; Gkrania-Klotsas et al. 2010; Uthamalingam et al. 2011; de Jager et al. 2012; Babio et al. 2013). Future studies are needed to address how much each WBC subtype affects morbidity and mortality risk in the ICU setting.
Although our results are informative, this study has certain limitations. First, the ICU design of the study limits the applicability of our conclusions to other settings, despite the abundance and detail of the data. Second, certain valuable variables were not obtained in the present study, such as the counts of intra-renal WBC and its subtypes; the counts of the secondary subtypes of WBC; and the levels of other inflammatory markers, including cytokine and chemokine, all of which may be additional predictors of outcomes. However, the WBC and WBC subtypes used in the present study are more clinically accessible examinations and thus may better fit the real clinical practice. Further studies addressing these limitations will be necessary in the future.
The present study is the first to have thoroughly evaluated the correlations of WBC and WBC subtype counts with AKI and mortality in critically ill patients. To date, there is no information on how to monitor and treat these markers in ICU patients at high risk of AKI. Furthermore, whether reducing counts of WBC or its subtypes significantly decreases the risk of AKI or mortality has not been determined. However, immune modulation is regarded as a promising therapy in the ICU setting, and related studies are ongoing. The present study will form the basis of later studies to address these issues.
Acknowledgements
This work was supported by a grant from the National Research Foundation of Korea (No. 2013R1A1A1012689).
Conflict of Interest
The authors declare no conflict of interest.
References
-
Abidi,
K.,
Belayachi,
J.,
Derras,
Y.,
Khayari,
M.E.,
Dendane,
T.,
Madani,
N.,
Khoudri,
I.,
Zeggwagh,
A.A. &
Abouqal,
R.
(2011) Eosinopenia, an early marker of increased mortality in critically ill medical patients. Intensive Care Med., 37, 1136-1142.
-
Babio,
N.,
Ibarrola-Jurado,
N.,
Bullo,
M.,
Martinez-Gonzalez,
M.A.,
Warnberg,
J.,
Salaverria,
I.,
Ortega-Calvo,
M.,
Estruch,
R.,
Serra-Majem,
L.,
Covas,
M.I.,
Sorli,
J.V. &
Salas-Salvado,
J.
(2013) White blood cell counts as risk markers of developing metabolic syndrome and its components in the predimed study. PLoS One, 8, e58354.
-
Bagshaw,
S.M.,
George,
C. &
Bellomo,
R.
(2008) Early acute kidney injury and sepsis: a multicentre evaluation. Crit. Care, 12, R47.
-
Burne,
M.J.,
Daniels,
F.,
El Ghandour,
A.,
Mauiyyedi,
S.,
Colvin,
R.B.,
O’Donnell,
M.P. &
Rabb,
H.
(2001) Identification of the CD4(+) T cell as a major pathogenic factor in ischemic acute renal failure. J. Clin. Invest., 108, 1283-1290.
-
Burne-Taney,
M.J.,
Ascon,
D.B.,
Daniels,
F.,
Racusen,
L.,
Baldwin,
W. &
Rabb,
H.
(2003) B cell deficiency confers protection from renal ischemia reperfusion injury. J. Immunol., 171, 3210-3215.
-
Chertow,
G.M.,
Burdick,
E.,
Honour,
M.,
Bonventre,
J.V. &
Bates,
D.W.
(2005) Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J. Am. Soc. Nephrol., 16, 3365-3370.
-
Clermont,
G.,
Acker,
C.G.,
Angus,
D.C.,
Sirio,
C.A.,
Pinsky,
M.R. &
Johnson,
J.P.
(2002) Renal failure in the ICU: comparison of the impact of acute renal failure and end-stage renal disease on ICU outcomes. Kidney Int., 62, 986-996.
-
Cunningham,
P.N.,
Dyanov,
H.M.,
Park,
P.,
Wang,
J.,
Newell,
K.A. &
Quigg,
R.J.
(2002) Acute renal failure in endotoxemia is caused by TNF acting directly on TNF receptor-1 in kidney. J. Immunol., 168, 5817-5823.
-
Day,
Y.J.,
Huang,
L.,
Ye,
H.,
Li,
L.,
Linden,
J. &
Okusa,
M.D.
(2006) Renal ischemia-reperfusion injury and adenosine 2A receptor-mediated tissue protection: the role of CD4+ T cells and IFN-gamma. J. Immunol., 176, 3108-3114.
-
de Jager,
C.P.,
Wever,
P.C.,
Gemen,
E.F.,
Kusters,
R.,
van Gageldonk-Lafeber,
A.B.,
van der Poll,
T. &
Laheij,
R.J.
(2012) The neutrophil-lymphocyte count ratio in patients with community-acquired pneumonia. PLoS One, 7, e46561.
-
Deitch,
E.A.
(1992) Multiple organ failure. Pathophysiology and potential future therapy. Ann. Surg., 216, 117-134.
-
Desquilbet,
L. &
Mariotti,
F.
(2010) Dose-response analyses using restricted cubic spline functions in public health research. Stat. Med., 29, 1037-1057.
-
Dragu,
R.,
Khoury,
S.,
Zuckerman,
R.,
Suleiman,
M.,
Mutlak,
D.,
Agmon,
Y.,
Kapeliovich,
M.,
Beyar,
R.,
Markiewicz,
W.,
Hammerman,
H. &
Aronson,
D.
(2008) Predictive value of white blood cell subtypes for long-term outcome following myocardial infarction. Atherosclerosis, 196, 405-412.
-
Ducloux,
D.,
Courivaud,
C.,
Bamoulid,
J.,
Vivet,
B.,
Chabroux,
A.,
Deschamps,
M.,
Rebibou,
J.M.,
Ferrand,
C.,
Chalopin,
J.M.,
Tiberghien,
P. &
Saas,
P.
(2010) Prolonged CD4 T cell lymphopenia increases morbidity and mortality after renal transplantation. J. Am. Soc. Nephrol., 21, 868-875.
-
Erlinger,
T.P.,
Tarver-Carr,
M.E.,
Powe,
N.R.,
Appel,
L.J.,
Coresh,
J.,
Eberhardt,
M.S. &
Brancati,
F.L.
(2003) Leukocytosis, hypoalbuminemia, and the risk for chronic kidney disease in US adults. Am. J. Kidney Dis., 42, 256-263.
-
Faubel,
S.,
Lewis,
E.C.,
Reznikov,
L.,
Ljubanovic,
D.,
Hoke,
T.S.,
Somerset,
H.,
Oh,
D.J.,
Lu,
L.,
Klein,
C.L.,
Dinarello,
C.A. &
Edelstein,
C.L.
(2007) Cisplatin-induced acute renal failure is associated with an increase in the cytokines interleukin (IL)-1beta, IL-18, IL-6, and neutrophil infiltration in the kidney. J. Pharmacol. Exp. Ther., 322, 8-15.
-
Felmet,
K.A.,
Hall,
M.W.,
Clark,
R.S.,
Jaffe,
R. &
Carcillo,
J.A.
(2005) Prolonged lymphopenia, lymphoid depletion, and hypoprolactinemia in children with nosocomial sepsis and multiple organ failure. J. Immunol., 174, 3765-3772.
-
Fried,
L.,
Solomon,
C.,
Shlipak,
M.,
Seliger,
S.,
Stehman-Breen,
C.,
Bleyer,
A.J.,
Chaves,
P.,
Furberg,
C.,
Kuller,
L. &
Newman,
A.
(2004) Inflammatory and prothrombotic markers and the progression of renal disease in elderly individuals. J. Am. Soc. Nephrol., 15, 3184-3191.
-
Gkrania-Klotsas,
E.,
Ye,
Z.,
Cooper,
A.J.,
Sharp,
S.J.,
Luben,
R.,
Biggs,
M.L.,
Chen,
L.K.,
Gokulakrishnan,
K.,
Hanefeld,
M.,
Ingelsson,
E.,
Lai,
W.A.,
Lin,
S.Y.,
Lind,
L.,
Lohsoonthorn,
V.,
Mohan,
V., et al.
(2010) Differential white blood cell count and type 2 diabetes: systematic review and meta-analysis of cross-sectional and prospective studies. PLoS One, 5, e13405.
-
Ishani,
A.,
Xue,
J.L.,
Himmelfarb,
J.,
Eggers,
P.W.,
Kimmel,
P.L.,
Molitoris,
B.A. &
Collins,
A.J.
(2009) Acute kidney injury increases risk of ESRD among elderly. J. Am. Soc. Nephrol., 20, 223-228.
-
Keane,
W.F.,
Brenner,
B.M.,
de Zeeuw,
D.,
Grunfeld,
J.P.,
McGill,
J.,
Mitch,
W.E.,
Ribeiro,
A.B.,
Shahinfar,
S.,
Simpson,
R.L.,
Snapinn,
S.M. &
Toto,
R.
(2003) The risk of developing end-stage renal disease in patients with type 2 diabetes and nephropathy: the RENAAL study. Kidney Int., 63, 1499-1507.
-
Kidney Disease: Improving Global Outcomes (KDIGO) acute kidney injury work group.
(2012) KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl., 2, 1-138.
-
Kinsey,
G.R.,
Sharma,
R.,
Huang,
L.,
Li,
L.,
Vergis,
A.L.,
Ye,
H.,
Ju,
S.T. &
Okusa,
M.D.
(2009) Regulatory T cells suppress innate immunity in kidney ischemia-reperfusion injury. J. Am. Soc. Nephrol., 20, 1744-1753.
-
Knaus,
W.A.,
Draper,
E.A.,
Wagner,
D.P. &
Zimmerman,
J.E.
(1985) APACHE II: a severity of disease classification system. Crit. Care Med., 13, 818-829.
-
Kuderer,
N.M.,
Dale,
D.C.,
Crawford,
J.,
Cosler,
L.E. &
Lyman,
G.H.
(2006) Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer, 106, 2258-2266.
-
Lee,
S.,
Huen,
S.,
Nishio,
H.,
Nishio,
S.,
Lee,
H.K.,
Choi,
B.S.,
Ruhrberg,
C. &
Cantley,
L.G.
(2011) Distinct macrophage phenotypes contribute to kidney injury and repair. J. Am. Soc. Nephrol., 22, 317-326.
-
Li,
L.,
Huang,
L.,
Sung,
S.S.,
Lobo,
P.I.,
Brown,
M.G.,
Gregg,
R.K.,
Engelhard,
V.H. &
Okusa,
M.D.
(2007) NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury. J. Immunol., 178, 5899-5911.
-
Madjid,
M.,
Awan,
I.,
Willerson,
J.T. &
Casscells,
S.W.
(2004) Leukocyte count and coronary heart disease: implications for risk assessment. J . Am. Coll. Cardiol., 44, 1945-1956.
-
Melnikov,
V.Y.,
Faubel,
S.,
Siegmund,
B.,
Lucia,
M.S.,
Ljubanovic,
D. &
Edelstein,
C.L.
(2002) Neutrophil-independent mechanisms of caspase-1- and IL-18-mediated ischemic acute tubular necrosis in mice. J. Clin. Invest., 110, 1083-1091.
-
Merino,
C.A.,
Martinez,
F.T.,
Cardemil,
F. &
Rodriguez,
J.R.
(2012) Absolute eosinophils count as a marker of mortality in patients with severe sepsis and septic shock in an intensive care unit. J. Crit. Care, 27, 394-399.
-
Praga,
M. &
Gonzalez,
E.
(2010) Acute interstitial nephritis. Kidney Int., 77, 956-961.
-
Turney,
J.H.
(1996) Acute renal failure: a dangerous condition. JAMA, 275, 1516-1517.
-
Uthamalingam,
S.,
Patvardhan,
E.A.,
Subramanian,
S.,
Ahmed,
W.,
Martin,
W.,
Daley,
M. &
Capodilupo,
R.
(2011) Utility of the neutrophil to lymphocyte ratio in predicting long-term outcomes in acute decompensated heart failure. Am. J. Cardiol., 107, 433-438.
-
Yang,
S.H.,
Lee,
J.P.,
Jang,
H.R.,
Cha,
R.H.,
Han,
S.S.,
Jeon,
U.S.,
Kim,
D.K.,
Song,
J.,
Lee,
D.S. &
Kim,
Y.S.
(2011) Sulfatide-reactive natural killer T cells abrogate ischemia-reperfusion injury. J. Am. Soc. Nephrol., 22, 1305-1314.
-
Ympa,
Y.P.,
Sakr,
Y.,
Reinhart,
K. &
Vincent,
J.L.
(2005) Has mortality from acute renal failure decreased? A systematic review of the literature. Am. J. Med., 118, 827-832.