2014 年 233 巻 1 号 p. 1-8
2014 年 233 巻 1 号 p. 1-8
Recent advances in technology have enabled the noninvasive evaluation of pulsatile hemodynamics in the central aorta; namely, central pressure and flow measurements. The central blood pressure represents the true load imposed on the heart, kidney and brain, and the central blood flow influences the local flow into these vital organs. An elevation of the central blood pressure has a direct, adverse impact on the target organ and, thus, the cardiovascular prognosis in patients with hypertension. A decrease in the central blood flow can cause organ dysfunction and failure. The central pressure and flow dynamics were conventionally regarded as unidirectional from the heart to the periphery. However, current evidence suggests that it should be recognized as a bidirectional interplay between the central and peripheral arteries. Specifically, the pressure pulse wave is not only transmitted forward to the periphery but also reflected backward to the central aorta. The flow pulse wave is also composed of the forward and reverse components. Aortic stiffening and arteriolar remodeling due to hypertension not only augment the central pressure by increasing the wave reflection but also may alter the central bidirectional flow, inducing hemodynamic damage/dysfunction in susceptible organs. Therefore, central hemodynamic monitoring has the potential to provide a diagnostic and therapeutic basis for preventing systemic target organ damage and for offering personalized therapy suitable for the arterial properties in each patient with hypertension. This brief review will summarize hypothetical mechanisms for the association between the central hemodynamics and hypertensive organ damage in the heart, kidney and brain.
For many years, blood pressure has generally been measured at the brachial artery, and the brachial blood pressure has been widely used for the diagnosis and treatment of hypertension. Consequently, the brachial pressure is often regarded as representative of the systemic blood pressure. In fact, however, the blood pressure shows considerable differences along the arterial tree, and it differs among the arterial sites at which the measurement is made (Avolio et al. 2009). For instance, there is a substantial pressure difference between the central aorta and brachial artery; the difference in the systolic maximum pressure reaches about 10-15 mmHg on average (McEniery et al. 2008; O’Rourke and Adji 2010). In addition, this aortic-brachial pressure difference varies considerably among individuals, and it occasionally exceeds 30 mmHg under certain circumstances, such as in young adolescents (O’Rourke et al. 2000) or during exercise (Rowell et al. 1968).
Hypertension is well-known as a risk factor for cardiovascular diseases. Nevertheless, previous investigations have proved no consistent associations between the brachial blood pressure and end-organ damage/dysfunction in the heart, brain and kidney. This has obscured hemodynamic mechanisms underlying hypertension-induced damage of target organs. However, based on the above-mentioned appreciable blood pressure difference between the arm and body trunk, it could be hypothesized that the central (aortic), rather than peripheral (brachial), hemodynamic alterations would have a more direct impact on the internal organs. Recent advances in non-invasive techniques with applanation tonometry have enabled the application of central hemodynamic assessments to clinical patients and more widely to the general population.
The present review discusses the association between the central hemodynamics and target organ damage in hypertension. This brief review focuses mainly on our recent data in an attempt to advance hypothetical mechanisms for hypertensive damage in the heart, kidney and brain.
Until quite recently, the central aortic pressure was measured only rarely because it required invasive (intra-arterial) catheterization. Currently the central pressure has been much more readily measurable in daily clinical practice, since noninvasive applanation tonometry was developed to record the radial (or carotid) pressure waveform (Kelly et al. 1989) and, in addition, the mathematical method (transfer function method) was established to estimate the aortic pressure waveform from the radial waveform (Karamanoglu et al. 1993).
The electrical transmission line (tube) theory has proposed that the central aortic blood pressure waveform is composed of two waves: the incident wave and reflected wave (O’Rourke and Taylor 1967; Murgo et al. 1981). The incident pressure wave, which is generated by cardiac ejection, travels forward along the arterial wall from the ascending aorta to the peripheral arteries at a speed termed the pulse wave velocity (PWV). When the incident wave encounters high impedance sites such as artery-arteriole junctions and arterial branching points, it is reflected and then travels backward to the ascending aorta. The reflected wave speed can be considered identical to the incident wave speed (Taylor 1966). Because the PWV is normally as fast as 5-10 m/s, the reflected wave arrives at the ascending aorta within the same cardiac cycle and it overlaps with the incident wave to boost the blood pressure (Murgo et al. 1980). This augmenting effect of wave reflection on the blood pressure is usually quantified by the augmentation index (Kelly et al. 1989).
As the aorta becomes stiffer with aging and/or hypertension, the incident pressure wave becomes greater on account of the decreased aortic distensibility (Mitchell et al. 2010). In addition, the resultant faster PWV causes earlier return of the reflected wave, which augments the aortic pressure in late systole to further increase the maximal systolic pressure and widen the pulse pressure (Namasivayam et al. 2009).
In the brachial artery as well, the reflected pressure returning from the lower body boosts the late-systolic pressure. In most cases (namely, radial augmentation index < 100%), however, this augmentation does not affect the maximum (early-)systolic and pulse pressures of the brachial artery, and it only appears as the second highest peak (or shoulder). Therefore, the wave reflection (arising from the lower body) can elevate only the aortic systolic and pulse pressures without changing the brachial peak systolic and pulse pressures.
Together with the wave reflection, the non-uniform elasticity of the arterial tree is also important in determining the blood pressure. Normally, the elastic aorta is more distensible than the muscular arteries. This normal stiffness gradient (as well as wave reflection) creates the pulse pressure amplification, namely the phenomenon that the pulse pressure increases from the central aorta to the peripheral muscular arteries in the upper and lower extremities (Wilkinson et al. 2001). With aging and/or hypertension, this stiffness gradient diminishes or even reverses owing to elastin degeneration (McEniery et al. 2007), resulting in a decrease in pulse amplification (McEniery et al. 2008).
Additionally, recent studies have emphasized the importance of the aortic reservoir function in determining the systolic pressure waveform (Sharman et al. 2009; Davies et al. 2010). The relative contribution of this reservoir pressure wave (in comparison with the reflected pressure wave) to late-systolic augmentation is currently a matter of debate (Mynard et al. 2012; Segers et al. 2012), while it has long been well-recognized that the diastolic pressure waveform is determined by the Windkessel (reservoir and recoil) function.
There is close interaction between the heart and vascular system. The vascular system plays not only a passive role of receiving the blood ejected from the heart but also an active role through its Windkessel function and by the reflection phenomenon. In healthy adolescents, the aortic incident pressure wave generated by the cardiac ejection is low in height, because the aorta is perfectly elastic with the reservoir function. In addition, the PWV is relatively slow, so the reflected wave returns in early diastole (rather than late systole). All these contribute beneficially to the cardiac work, since the lowered aortic systolic pressure maintains low cardiac afterload while the enhanced early-diastolic pressure serves to increase coronary flow. By contrast, in the case of elderly patients with aortosclerosis, the aortic incident wave is higher and the reflected wave returns earlier in systole, so the cardiac afterload is markedly increased while the coronary flow is reduced (O’Rourke and Hashimoto 2007).
Left ventricular hypertrophy is known as a strong predictor of heart failure and coronary artery disease. Recent observations have suggested that it has a closer association with the central blood pressure than with the brachial blood pressure (Hashimoto et al. 2006; Roman et al. 2010). Our data in patients with untreated hypertension indicate that the development of hypertrophy is attributable to the extra workload of the left ventricle, which is wasted to generate cardiac outflow against the afterload augmented by the reflected wave (Hashimoto et al. 2008b). In addition, the regression of left ventricular hypertrophy during antihypertensive treatment is considered proportional to the reductions in the aortic augmentation index and reflection magnitude (de Luca et al. 2004; Hashimoto et al. 2007b, 2008c). Another study showed that the casual (office) central pressure indices can better predict the regression than the home brachial pressure (Hashimoto et al. 2007a). These data suggest that left ventricular hypertrophy is a compensating reaction to the central pressure overload as exacerbated by the peripheral wave reflection, and it is (at least partially) reversible by normalizing the central hemodynamics.
Manifestations of cardiac damage induced by central hemodynamic abnormalities are not confined to left ventricular hypertrophy. For instance, the aortic augmentation index and augmented pressure are shown to be associated with the prevalence and severity of coronary artery disease (Weber et al. 2004), suggesting their contribution to coronary atherogenesis. Aortic pressure augmentation due to wave reflection could cause myocardial ischemia by increasing the left ventricular wasted effort and ejection duration, even in the absence of overt coronary stenosis (Nichols et al. 2013). As summarized in previous reviews (Vlachopoulos et al. 2010; Hashimoto and Ito 2012), several prospective studies conducted on coronary artery disease patients have demonstrated that the central pulse pressure and augmentation index predict future cardiovascular events, and the predictive ability is superior to (or independent of) the brachial pressure (Chirinos et al. 2005; Weber et al. 2005, 2010; Jankowski et al. 2008).
The kidney is a high-flow organ, which receives blood corresponding to 20-25% of the cardiac output. This high flow is usually maintained by the low resistance of the renal microvasculature to facilitate glomerular filtration. The glomeruli located between the afferent and efferent arterioles are composed of capillary vessels, which are by nature fragile in structure. These unique characteristics in terms of structure and function make the kidney highly susceptible to central hemodynamic alterations.
Micro-puncture studies in normal rats demonstrated that the intra-glomerular pressure approximates 60/40 mmHg in peak systole/end-diastole (Drumond and Deen 1991). Accordingly, the glomerular capillaries are usually exposed to much higher and more pulsatile pressure than the capillaries of other organs (such as skeletal muscles), since the latter normally show a lower and nearly steady pressure (≈ 15 mmHg) (Shore 2000). Fig. 1A illustrates an estimate of the gradual change in the pulsatile pressure from the central aorta to intrarenal microvessels likely to be found in a normal young subject with an elastic aorta. The pressure pulsation in the central aorta travels, with relatively little damping, through the arcuate arteries, afferent arterioles, and even deeply into the glomerular capillaries.
When the aortic pressure pulsation (ie, aortic pulse pressure) increases with aortic stiffening or hypertension, the increased pulsation is transmitted through the intrarenal middle-sized arteries down to the glomerular capillaries on account of the low resistance properties of the renal microvasculature (Fig. 1B). A resultant increase in the repetitive tensile stress imposed on the capillary wall leads to glomerular hyperfiltration and endothelial damage, both of which induce (micro)albuminuria (Fig. 2). In fact, a recent study has demonstrated that a higher aortic pulse pressure is independently associated with the presence of albuminuria in patients with hypertension (Hashimoto and Ito 2011). This study agrees well with previous studies showing significant associations of the aortic PWV and augmentation index with the urinary albumin excretion (Tsioufis et al. 2003; Smith et al. 2005). Moreover, the aortic pulse pressure has been shown to correlate with proteinuria and serum creatinine more closely than the peripheral (femoral) pulse pressure in patients with coronary risk (Temmar et al. 2010). All these data indicate an etiological role of increased central pressure pulsation in renal microvascular damage (“pressure” hypothesis) (Mitchell 2004; Safar 2004).
Another mechanism responsible for renal microvascular damage could be mediated through blood flow alteration (Fig. 2). In studies examining the intrarenal flow velocity waveform with duplex ultrasound (Pontremoli et al. 1999; Hashimoto and Ito 2011), it has been demonstrated that (micro)albuminuria is predictable by the renal resistive index (RI). This means that intrarenal flow pulsation is related to glomerular injury, because RI is usually calculated from the equation of [RI = 1 – (minimum velocity) ÷ (maximum velocity)] and therefore it originally quantifies the flow pulsation. More specifically, the association between renal RI and albuminuria can be explained based on the mechanical basis that the increased pulsatile flow produces repetitive, frictional shear stress to the glomerular capillaries, which can eventually lead to endothelial injury resulting in albuminuria (“flow” hypothesis) (O’Rourke and Hashimoto 2007).
It is important to note here that the renal RI strongly depends on the aortic pulse pressure and PWV (Hashimoto and Ito 2011). This dependence reflects the fact that the intrarenal flow pulsation is determined by the aortic pressure pulsation (Mills et al. 1970; Tublin et al. 1999). The dependence of renal RI on the central hemodynamics has been suggested also by a recent study of renal transplant recipients, which showed an association between renal RI and recipient age (related to aortic stiffness) but not renal allograft histologic features (Naesens et al. 2013). Alternatively, if adopting the conventional view that the RI measures peripheral resistance (Norris and Barnes 1984), one could speculate that increased pressure pulsation transmitted to the afferent arterioles directly stimulates the myogenic response that causes arteriolar constriction (Bidani et al. 2009) and thus elevates the RI (Fig. 2).
The central pulse pressure seems particularly important for patients with chronic kidney disease (CKD). Townsend et al. reported that each 10 ml/min per 1.73 m2 decrement in the estimated glomerular filtration rate was associated with an increase in the central pulse pressure of ≈ 2.5 mmHg in a large patient cohort of mild-to-moderate CKD (Townsend et al. 2010). Briet et al. showed that the central pulse pressure was an independent predictor of the progression to end-stage renal disease in CKD patients (Briet et al. 2011). Furthermore, some prospective studies have investigated the predictive ability of central pressure indices for cardiovascular prognosis in patients with end-stage renal disease (London et al. 2001; Safar et al. 2002; Covic et al. 2006) or in renal transplant recipients (Verbeke et al. 2011). Most of the studies have validated that the central pulse pressure and augmentation index predict future cardiovascular events more accurately than, or independently of, the brachial pressure (Hashimoto and Ito 2012).

Estimate of the change in pulsatile pressure from the central aorta to intrarenal microvasculature in subjects with an elastic aorta (A) and with a stiff aorta (B).
art. indicates artery. Note that the intraglomerular pulsatile pressure is markedly elevated in a subject with a stiff aorta.
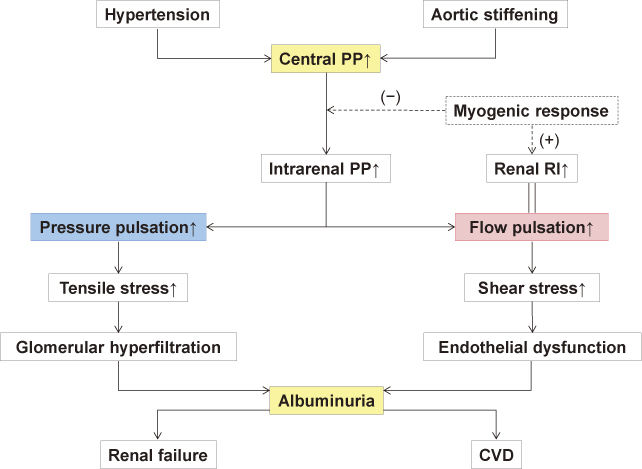
Hypothetical mechanism for renal microvascular damage induced by hypertension and aortic stiffening.
PP indicates pulse pressure; RI, resistive index; CVD, cardiovascular disease.
Refer to the text for details.
The cerebral vasculature has similar characteristics to the renal vasculature in terms of structure and function. Analogous with the kidney, the cerebral microvasculature receives high and pulsatile blood flow owing to its low resistance and impedance, as manifest by the unidirectional flow features (without reverse flow) of the cerebral arteries (Liu et al. 2013). Structurally, the relationship between the large middle cerebral arteries and small perforating arteries of the brain resembles the relationship between the arcuate arteries and juxtamedullary afferent arterioles of the kidney in that the tiny arterioles directly branch off from the large arteries within a short distance (Ito 2012). Therefore, it seems reasonable to assume that, like the glomerular capillaries, the cerebral perforating arteries are particularly susceptible to central hemodynamic alterations.
Currently available data do support this assumption. The central blood pressure has been shown to be associated with cerebral lacunar infarction (Ochi et al. 2010), which occurs frequently in the areas of blood supply governed by the perforating arteries. Likewise, the central pressure is associated with white matter hyperintensity lesions (seen on brain MRI), which are manifestations of cerebral microvascular damage, and this association is stronger than that with the brachial pressure (Shrestha et al. 2009). The fact that the PWV is an indicator of lacunar infarction and white matter lesions (Hashimoto et al. 2008a; Henskens et al. 2008; Mitchell et al. 2011; Wohlfahrt et al. 2014) can be considered evidence that aortic stiffening underlies these associations between the increased central pressure and cerebral microvascular damage. In addition, a recent study has found an independent association between the cerebral blood flow pulsatility and white matter lesions (Webb et al. 2012), suggesting that the flow as well as pressure pulsations are responsible for microvascular damage in the brain (Hirata et al. 2006; Adji et al. 2011; Mitchell et al. 2011), as in the kidney.
In contrast to microvascular damage, few studies have been conducted on macrovascular brain damage from the perspective of central hemodynamics. However, recent investigations are already suggesting the possibility that diastolic reverse flow in the proximal descending aorta causes retrograde plaque embolism leading to major stroke (Harloff et al. 2009), and this aortic reverse flow increases with aortic stiffening (Hashimoto and Ito 2013).
As mentioned earlier, the pulse and maximum systolic pressures normally increase gradually from the central aorta toward the peripheral muscular arteries. This phenomenon is generally termed the pulse pressure amplification (Avolio et al. 2009). Due to this amplification, the blood pressure during late systole and early diastole is much higher in the peripheral than central arteries when measured simultaneously (Rowell et al. 1968). The amplification ratio was reported to be approximately 130% between the central aorta and femoral artery in a hypertensive population (Hashimoto and Ito 2010).
A recent investigation has revealed that the aorta-to-femoral pulse pressure amplification is positively correlated with the reverse flow in the femoral artery (Hashimoto and Ito 2010). This finding can be explained by the hypothetical mechanism that the aorta-to-femoral pressure difference is primarily responsible for the bidirectional (triphasic) waveform of the femoral flow (McDonald 1955). As shown in Fig. 3, this mechanism is likened to water on a seesaw, assuming that the direction and velocity of water flow are determined by the slope of the seesaw (which corresponds to the pressure gradient). Accordingly, in the early systole when the aortic pressure is higher than the femoral pressure, the blood flows forward in accordance with this positive pressure gradient (Fig. 3A). Subsequently, in the late systole and early diastole when the aortic pressure is lower than the femoral pressure, the blood flows in reverse, in accordance with the inverse pressure gradient (Fig. 3B). In the mid-diastole when the aortic pressure returns to being higher, the blood flows forward again (Fig. 3C). This hypothesis is consistent with actual observations, although additional effects of arterial distensibility (reservoir function) and blood inertia must also be taken into account. Of particular note in this study is that, as the pulse pressure amplification decreases with aortic stiffening, the reverse flow also decreases proportionally (Hashimoto and Ito 2010).
Bogren et al. previously speculated that the reverse flow in the lower body may supply diastolic flow into internal abdominal organs such as the kidney (Bogren and Buonocore 1994). This agrees with our recent study showing an inverse correlation between the femoral reverse flow index and renal RI (Hashimoto and Ito 2011), which suggests that the femoral (and thus infra-renal aortic) reverse flow contributes positively to the renal diastolic flow. Of further importance, as pulse amplification deceases due to aortosclerosis, the renal diastolic flow (relative to the systolic flow) is also reduced. This clearly indicates that normal pulse pressure amplification is essential to maintain the diastolic flow into the kidney.

Hypothetical mechanism for the association between alternate pressure gradients and bidirectional (triphasic) flow at the femoral artery (seesaw theory).
Ao indicates aorta; Fem, femoral artery; FWD, forward flow; REV, reverse flow.
Upper panels: The slope of the seesaw (shown in red) represents pressure gradient, and the arrow (in green) represents flow velocity and direction. Changes in pressure gradient and flow velocity are shown in chronological order from early systole (A), late systole/early diastole (B) to mid-diastole (C).
Lower panels: Femoral flow waveform is depicted in time domain, with the horizontal dotted line as zero flow. The green circle represents femoral flow at the corresponding time.
Refer to the text for more details.
The currently available evidence mentioned above suggests that central hemodynamics is deeply involved in the pathophysiology of hypertensive target organ damage. It is also becoming increasingly evident that the central blood pressure-guided treatment of hypertension can contribute to a better cardiovascular prognosis and more appropriate use of antihypertensive medication (Williams et al. 2006; Sharman et al. 2013). In conclusion, central hemodynamic monitoring has great potential to provide a new diagnostic and therapeutic basis for preventing systemic target organ damage and offering personalized therapy suitable for the arterial properties in each individual patient with hypertension.
The author is sincerely grateful to Professors Sadayoshi Ito and Michael F. O’Rourke for their mentorship and Ms. Saki Asano for her secretarial assistance.
The author declares no conflict of interest.