2014 Volume 233 Issue 2 Pages 113-122
2014 Volume 233 Issue 2 Pages 113-122
Microglia are the main immunocompetent and phagocytic cells in Alzheimer’s disease (AD). Bone marrow-derived microglia have been demonstrated to be more effective in antigen presentation and phagocytosis than inherent microglia in AD. Thus, microglia have received much attention in the pathogenesis of AD. The herbal medicine Juzen-taiho-to (JTT) has been reported to reduce β-amyloid (Aβ) burden in the mouse brain of an AD model. In this study, we explored the effects of JTT on the migration and differentiation of bone marrow-derived cells in the mouse brain of acutely induced AD. To chase bone marrow-derived cells, we made a chimeric mouse line in C57BL/6 by transplanting fresh bone marrow cells, isolated from the transgenic mice expressing enhanced green fluorescent protein gene. The chimeric mice were orally administrated with JTT or distilled water, and were left untreated or given intrahippocampal injection of fibrillar Aβ 1-42 (fAβ42) or vehicle. In the hippocampus of the vehicle-injected mouse, JTT treatment for 37 days caused a significant increase in the number of microglial cells. In the fAβ42-injected mouse hippocampus, a larger number of bone marrow-derived cells were detected in JTT-treated mice than control mice in the non-neighboring regions of the fAβ42-injected site but not around the injected site. These results suggest that JTT might contribute to the reduction of Aβ burden and the immune surveillance in non-pathological as well as pathological brain regions. The results also implicate the therapeutic potential of JTT in AD.
It has been reported that innate immunity, especially that involving macrophage function diminishes with age as well as in patients with Alzheimer’s disease (AD). In recent years, microglia, which are derived from bone marrow stem cells (BMSC), have become a hot topic. Many studies gave evidence that BMSC could penetrate through the blood-brain barrier (BBB) into the central nervous system (CNS), and differentiate into functional microglia (Hess et al. 2004; Simard and Rivest 2004; Malm et al. 2005, 2010; Massengale et al. 2005). However, it remains controversial whether or not BMSC could enter the normal brain. Vallieres and Sawchenko (2003) reported that only a small number of BMSC could enter the brain parenchyma and then differentiate into microglia, and that these bone marrow-derived microglia (BMDM) were found only in the cerebellum. While other studies confirmed that BMSC could not differentiate into the parenchymal microglia (Fricker et al. 1999; Gage 2000), Simard and Rivest (2004) reported that BMDM could be found throughout the mouse brain parenchyma with integral BBB. In other studies, BMDM were found more easily to be chemoattracted into the brain with neuropathy (Villeneuve et al. 2005; Simard et al. 2006; Priller et al. 2006), although the precise mechanism has not been entirely cleared yet.
Simard et al. (2006) reported that after bone marrow transplantation, 6-month-old amyloid precursor (APP)/presenilin 1 (PS1) double transgenic AD mice generated new microglia, and these microglia were attracted around the senile plaques specifically. More than 90% of the microglia around the senile plaques were BMDM. These BMDM played a role in delaying disease progression by phagocytosing β-amyloid protein (Aβ) (Simard et al. 2006). They also found that, compared with inherent microglia these BMDM expressed a higher level of CD11c, a marker for more efficient antigen-presenting and phagocytic cells. Thus, transplantation of bone marrow cells could be expected as a new strategy in AD therapy.
Macrophage colony-stimulating factor (M-CSF), granulocyte colony-stimulating factor (G-CSF), and stem cell factor plus G-CSF were found to augment BMDM that degrade Aβ efficiently (Boissonneault et al. 2009; Sanchez-Ramos et al. 2009; Li et al. 2011). Juzen-taiho-to (JTT, Shi-Quan-Da-Bu-Tang in Chinese), an herbal medicine, has traditionally been administrated to patients with anemia, anorexia, cancer, or fatigue. Pharmacologically, JTT contains various immunomodulatory substances, and it is known to have lipopolysaccharide-like functions and exhibit anti-tumor effects by activating immune responses and enhancing phagocytosis (Hisha et al. 1997; Kamiyama et al. 2005). Our previous study reported that JTT activated microglia and induced microglial proliferation and activation (Liu et al. 2008). Furthermore, bone marrow-derived macrophages from JTT-treated mice showed enhanced phagocytosis of fibrillar Aβ1-42 (fAβ42) (Liu et al. 2008). Later, Hara et al. (2010) reported that orally administrated JTT reduced Aβ burden in the mouse brain of an AD model by activating bone marrow-derived macrophages. They speculated that these macrophages crossed the BBB, differentiated into microglia, and phagocytosed fibrillar Aβ in senile plaques.
In this study, we established chimeric mice by transplanting bone marrow cells from transgenic mice expressing enhanced green fluorescent protein (EGFP) gene (Simard et al. 2006). Using the chimeric mice, we examined whether JTT could increase phagocytosis of intrahippocampally injected fAβ42 by BMDM and reduce the lesion by promoting the homing of BMDM.
Heterozygous transgenic mice with H-2b genetic background, expressing EGFP under control of the chicken β-actin promoter and cytomegalovirus enhancer were initially obtained from The Jackson Laboratory (Okabe et al. 1997). The strain of the mice was C57BL/6-Tg (ACTB-EGFP) 10sb/J. These EGFP mice were mated with C57BL/6 mice, and 3- to 5-month-old both sex mice were used as the donor of bone marrow transplantation. Twelve-month-old female C57BL/6 wild-type mice were used as the recipient of bone marrow transplantation. This experiment was performed at the National Institute for Longevity Sciences, National Center for Geriatrics and Gerontology in Obu, Japan, under the institutional guidelines and approval of the institute’s ethical committee for animal experiments. 16 recipient mice were randomly divided into four groups.
ReagentsSynthetic human Aβ1-42 peptide (Aβ42) was purchased from Peptide Institute, Inc. (Osaka, Japan). To obtain fAβ42, Aβ42 was dissolved in DMSO and then in Dulbecco’s PBS (DPBS, 300 μM), followed by incubating at 37°C for 7 days. Rabbit anti-ionized calcium binding adaptor molecule 1 (Iba1) polyclonal antibody was purchased from Wako Pure Chemicals Industries, Inc. (Osaka, Japan), mouse anti-human Aβ monoclonal antibody 4G8, mouse anti-neuron-specific nuclear protein monoclonal antibody (NeuN) and chicken anti-green fluorescent protein (GFP) polyclonal antibody were from Chemicon International, Inc. (Temecula, CA, USA), rabbit anti-GFP polyclonal antibody was from MBL International (Woburn, MA, USA), Cy3-conjugated mouse anti-glial fibrillary acidic protein (GFAP) monoclonal antibody was from Sigma-Aldrich (Saint Louis, MO, USA), PE-conjugated rat anti-mouse CD11b monoclonal antibody was from BD Biosciences Pharmingen (San Jose, CA, USA). Optimum cutting temperature compound (O.C.T.) was from Sakura Finetechnical (Tokyo, Japan). ABC Kit and diaminobenzidine (DAB) substrate kit for peroxidase were from Vector Laboratory, Inc. (Burlingame, CA, USA).
Preparation of JTT-containing distilled waterJTT, purchased from Tsumura and Co (Tokyo, Japan), was composed of 10 medical plants. JTT was prepared as follows; a mixture of Astragali Radix (3.0 g), Cinnamomi Cortex (3.0 g), Angelicae Radix (3.0 g), Paeoniae Radix (3.0 g), Cnidii Rhizoma (3.0 g), Rehmanniae Radix (3.0 g), Ginseng Radix (3.0 g), Atractylodis Lanceae Rhizoma (3.0 g), Poria (3.0 g), and Glycyrrhizae Radix (1.5 g) was added to 285 ml of water, and extracted at 100˚C for 1 h. The extracted solution was filtered and spray-dried to obtain the dry extract powder (2.3 g).
According to the previous studies (Ikehara et al. 1992; Dai et al. 2001; Chino et al. 2005), JTT extract powder was dissolved in distilled water by stirring at a concentration of 12.5 mg/ml for 1 h at room temperature. After dissolved, JTT was sonicated for 30 min (Branson Sonifier 250, USA), and centrifuged at 3,000 rpm for 10 min (J6-HC Centrifuge, Beckman Coulter, USA) to remove the insoluble materials. The supernatant was then filtered with a disposable syringe filter with a 5-μm PVDF membrane (Millipore, Cork, Ireland) and stored at 4°C.
Experimental protocolThe experimental protocol is shown in Fig. 1. All mice were fed with regular food for 4 weeks after bone marrow transplantation.
Eight mice were sacrificed after 37 days; 4 mice (JTT group) received JTT (12.5 mg/ml; the average dose was 2 g/kg/day), whereas the other 4 mice (control group) received distilled water.
Other 8 mice were similarly given JTT (JTT group, n = 4) or distilled water (control group, n = 4) for 4 weeks, then were injected with fAβ42 or DPBS intrahippocampally, and sacrificed 9 days after.

Experimental protocol.
After whole body irradiation, bone marrow cells from EGFP mice were transplanted into B6 mice, and the mice were then fed with regular food for 4 wks without any treatment (no Tx). After that, 8 chimeric mice were treated with JTT or distilled water for 37 days, then the blood and brains were examined. Other 8 mice were treated with either JTT or distilled water for 4 wks, then fAβ42 was injected into the hippocampus of one side and Dulbecco’s PBS into the other side. After further 9 days treatment with JTT or distilled water, their brains were examined histochemically.
According to the published paper (Simard et al. 2006), all mice were exposed to 10-gray whole body irradiation using an X-ray source (MBR-1520A-2, Hitachi, Japan). The next day, the animals were injected via a femoral vein with 5 × 106 bone marrow cells freshly collected from EGFP mice. The cells were aseptically harvested by flushing femurs and tibias with DPBS containing 2% fetal bovine serum (DPBS-FBS). The samples were combined, filtered through a 40-μm nylon mesh, centrifuged, and passed through a 25-ga needle. Recovered cells were resuspended in DPBS at a concentration of 5 × 106 viable nucleated cells per 200 μl. Irradiated mice transplanted with this suspension were housed in autoclaved cages and treated with antibiotics (0.2 mg trimethoprim and 1 mg sulfamethoxazole/ml of distilled water) for 7 days before and 2 weeks after irradiation.
Establishment of a fAβ42-injected mouse modelAnimals were anesthetized with pentobarbital (35 mg/kg), and the sites of injections were stereotaxically reached (SR-6N, Narishige Scientific Instrument Lab., Tokyo, Japan). For intrahippocampal injections of fAβ42, the coordinates from the bregma were +2.5 mm anteroposterior, −1.8 mm lateral, and −2 mm dorsoventral. Two microliter of solution containing fAβ42 (300 μM in DPBS) was delivered over a period of 4 min with a 10-μl micro-syringe. Then two microliter of DPBS was injected into the other side. The mice were housed up to four animals per cage and then sacrificed at the time point mentioned above.
Flow cytometric assayFlow cytometric assay was used to detect the GFP+ cells and CD11b+ cells in blood samples. In detail, the mice were anesthetized by inhalation of diethyl ether, then about 200 μl of blood samples were taken from the heart, collected in centrifuge tubes containing 25 mM EDTA, and mixed thoroughly. After red blood cells were lysed, the remaining blood cells were washed with FACS buffer (DPBS containing 4% FBS and 0.1% sodium azide), then about 50 μl of single-cell suspension was made for each sample. The samples were then incubated with PE-conjugated rat anti-mouse CD11b monoclonal antibody for 30 min on ice for cell surface staining. After washed three times with FACS buffer to remove the free antibody and marked the dead cells with propidium iodide (PI), the samples were examined by FACSCalibur flow cytometer (BD, Franklin Lakes, NJ, USA) and analyzed using the CellquestTM software (BD immunocytometry system, CA, USA).
Preparation of frozen brain sectionsTo collect the brain tissues, mice were anesthetized by inhalation of diethyl ether. After blood samples were taken from hearts, mice were sacrificed by cervical dislocation. The brains were rapidly removed from the skulls, post-fixed in 4% paraformaldehyde overnight at 4°C, and then washed with PBS, dehydrated with 10%, 20% and 30% sucrose diluted in PBS, and embedded in O.C.T.. The frozen sections were cut coronally with Leica CM 1850 cryostat microtome (Leica Microsystem Nussloch GmbH, Nussloch, Germany) at 10 μm in thickness. The slices were stored at −80°C.
Detection of GFP+ cells or Iba1+ cells by immunohistochemistrySections were rinsed with PBS before incubation in 0.3% H2O2 in methanol for 30 minutes to block endogenous peroxidase. Then sections were washed with PBS and incubated in blocking buffer (PBS containing 0.4% Triton X-100, 2% bovine serum albumin (BSA) and 10% normal goat serum) for 1 h. Using the same buffer solution, the sections were then incubated for 1 h in primary antibody (anti-GFP or anti-Iba1, 1:500) at room temperature. Anti-Iba1 antibodies are commonly used for detecting activated microglia (Ohsawa et al. 2004). The sections were then rinsed with PBS, followed by 1-h incubation in relevant biotin-conjugated secondary antibody. After PBS washes, sections were stained by the avidin-biotin HRP/DAB method.
Detection of GFP+ cells around the fAβ42-injected site by immunofluorescence stainingSections were washed with PBS and incubated in blocking buffer (PBS containing 0.4% Triton X-100, 2% BSA and 10% normal donkey serum) for 1 h. Using the same buffer solution, the sections were then incubated for 1 h in primary antibody (anti-GFP or anti-4G8, 1:500) at room temperature. The sections were then rinsed with PBS, followed by 1-h incubation in fluorochrome-conjugated secondary antibody (Alexa 488-conjugated anti-rabbit IgG and Alexa 594-conjugated anti-mouse IgG, 1:500, Molecular Probes). After PBS washes, sections were coverslipped with antifade medium, then images were projected from an Olympus IX70 microscope equipped with appropriate filters.
Phenotype assay for GFP+ cells by immunofluorescence stainingSections were washed with PBS and incubated in blocking buffer (PBS containing 0.4% Triton X-100, 2% BSA and 10% normal secondary antibody relevant serum) for 1 h. Using the same buffer solution, the sections were then incubated for 1 h in primary antibody (anti-GFP and anti-Iba1, 1:500; anti-GFP and anti-NeuN, 1:500; anti-GFP, 1:500) at room temperature. Anti-NeuN antibodies are commonly used for labeling neurons (Atmaca et al. 2014). The sections were then rinsed with PBS, followed by 1-h incubation in fluorochrome-conjugated secondary antibody (Alexa 488-conjugated anti-chicken IgG and Alexa 594-conjugated anti-rabbit IgG, 1:500; Alexa 488-conjugated anti-rabbit IgG and Alexa 594-conjugated anti-mouse IgG, 1:500; Alexa 488-conjugated anti-rabbit IgG and Cy3-conjugated anti-GFAP, 1:500). After PBS washes, sections were coverslipped with antifade medium, then images were projected from the Olympus IX70 microscope.
Statistical analysisAll results were expressed as the means ± s.d. The statistical significance was determined by two-tailed Student’s t-test.
To verify the ability of transplanted bone marrow cells to generate new blood cells, we checked the ratio of GFP+ cells and CD11b+ cells in the mouse blood using flow cytometric assay. As shown in Fig. 2A and B, the percentages of GFP+ cells out of total white blood cells were 79.9 ± 8.6% and 79.7 ± 11.3% in control group and JTT-treated group, respectively, and there was no significant difference between two groups. Compared with the percentage of GFP+ blood cells in EGFP mice at the same age (76.9 ± 6.1%, n = 4), there was also no significant difference (data not shown). These results confirmed that establishment of a chimeric mouse model was successful; more than 79% white blood cells in chimeric mice were generated from the transplanted bone marrow cells.
To determine whether JTT promoted the proliferation of blood monocyte-macrophage lineage cells, we compared the CD11b+ cells in the mouse blood (Fig. 2A and C). The percentages of CD11b+ cells out of total white blood cells were 13.9 ± 2.0% and 21.1 ± 5.5% in control group and JTT-treated group, respectively, showing a significant statistical difference between two groups (p = 0.049). These results showed that JTT could promote the proliferation of blood monocyte-macrophage lineage cells.

Identification of GFP+ cells and CD11b+ cells in the blood of chimeric mice by flow cytometry. Bone marrow cells from EGFP mice were transplanted to B6 mice, and the ability of transplanted bone marrow cells to generate new blood cells was examined. (A) Identification of GFP+ cells and CD11b+ cells in the mouse blood by flow cytometry; control (left) and JTT (right). (B) Proportion of GFP+ cells in total white blood cells in the mouse blood. (C) Proportion of CD11b+ cells in total white blood cells in the mouse blood. #p < 0.05 vs. control group analyzed by two-tailed Student’s t-test.
The frozen brains were cut into 10-μm coronary sections from olfactory bulb to medulla oblongata, and the sections at every 200 μm were chosen. The self-fluorescence of GFP was observed by fluorescence microscope, and then sections from different brain regions were chosen and were further verified by anti-GFP staining. The results showed that GFP+ cells were found occasionally in the cerebellum in both control group and JTT-treated group (Fig. 3A), suggesting that JTT did not promote proliferation of GFP+ cells in normal brain.
Then, we chose three coronary sections from each mouse hippocampus for staining with anti-Iba1 antibody. As shown in Fig. 3B and C, compared with control group, JTT increased the number of inherent microglia in normal hippocampus, showing significant difference between two groups (51.6 ± 8.1 in control group, 61.9 ± 12.1 in JTT-treated group, p = 0.022).

Detection of GFP+ cells or Iba1+ cells in the brain parenchyma of chimeric mice. Bone marrow cells from EGFP mice were transplanted in B6 mice, and GFP+ or Iba1+ cells in the brain parenchyma were examined by immunohistochemical staining. (A) GFP+ cells in the mouse cerebellum, as pointed by arrows. (B) Iba1+ cells in the hippocampus. (C) The number of Iba1+ cells in mouse hippocampus per × 20 field. n = 12. Scale bar, 200 μm. #p < 0.05 vs. control group analyzed by two-tailed Student’s t-test.
As shown in Fig. 4, using immunofluorescence double staining with anti-Aβ 4G8 and anti-GFP antibody, we found a large number of GFP+ cells gathered within and around the fAβ42-injected site in both groups. Since the infiltrated cells were so dense, it was difficult to count and compare the number of infiltrated cells. We also found GFP+ cells gathered within and around the DPBS-injected site (Fig. 4D and H), but the numbers were much fewer, suggesting that Aβ but not needle injury per se attracted GFP+ bone marrow cells.

Detection of GFP+ cells in the fAβ42-injected mouse hippocampus by immunofluorescence double staining. A-D, Control group; E-H, JTT group; Green, GFP; Red, Aβ; scale bar, 100 µm. (A and E) The sections were stained with antibody directed against GFP in the fAβ42-injected side. (B and F) The sections were stained with antibody directed against 4G8 in the fAβ42-injected side. Arrow-1, fAβ42 injection site. (C and G) The superimposing fluorescence images of A/B and E/F, respectively. (D and H) The sections were stained with antibody directed against GFP in the Dulbecco’s PBS-injected side. Arrow-2, Dulbecco’s PBS injection site. Much fewer but significant number of GFP+ cells were migrated in the injection site, suggesting that injury per se also attracts GFP+ bone marrow cells.
In view of the results above, it was difficult to get an accurate number of GFP+ cells around the fAβ42-injected site. Accordingly, we chose 3 hippocampal sections from each mouse, which were separated at least 200 μm from the injection site. We found that there were also a large number of GFP+ cells in the non-neighboring regions of fAβ42-injected site in both groups (Fig. 5A). The GFP+ cells in the non-neighboring regions of fAβ42-injected site (as shown in Fig. 5A) were counted and compared between two groups (Fig. 5B). The GFP+ cells were significantly increased in JTT-treated group compared with control group (p = 0.027).

Detection of GFP+ cells in non-neighboring regions of the fAβ42-injected site by immunohistochemical staining. (A) GFP+ cells in whole coronary sections: A-1, Control mouse, A-2, JTT-treated mouse; Arrow-1, fAβ42 injection site; Arrow-2, Dulbecco’s PBS injection site. Boxes indicate the area where we counted GFP+ cells. (B) GFP+ cells in non-neighboring regions of the fAβ42-injected side (in the boxes in Fig. 5A) were counted under magnification. As shown, the number of GFP+ cells was significantly increased in JTT-treated mice. n = 12, each. Scale bar, 2.0 mm. #p < 0.05 vs. control group analyzed by two-tailed Student’s t-test.
By immunofluorescence double staining, we confirmed that in either JTT-treated group or control group, and in either around or apart from the fAβ42-injected site, almost all the GFP+ cells showed the microglia phenotype (Iba1+), but not the neuron phenotype (NeuN+) or astrocyte phenotype (GFAP+). Brain sections of the fAβ42-injected side in JTT-treated group were selected, as shown in Fig. 6.
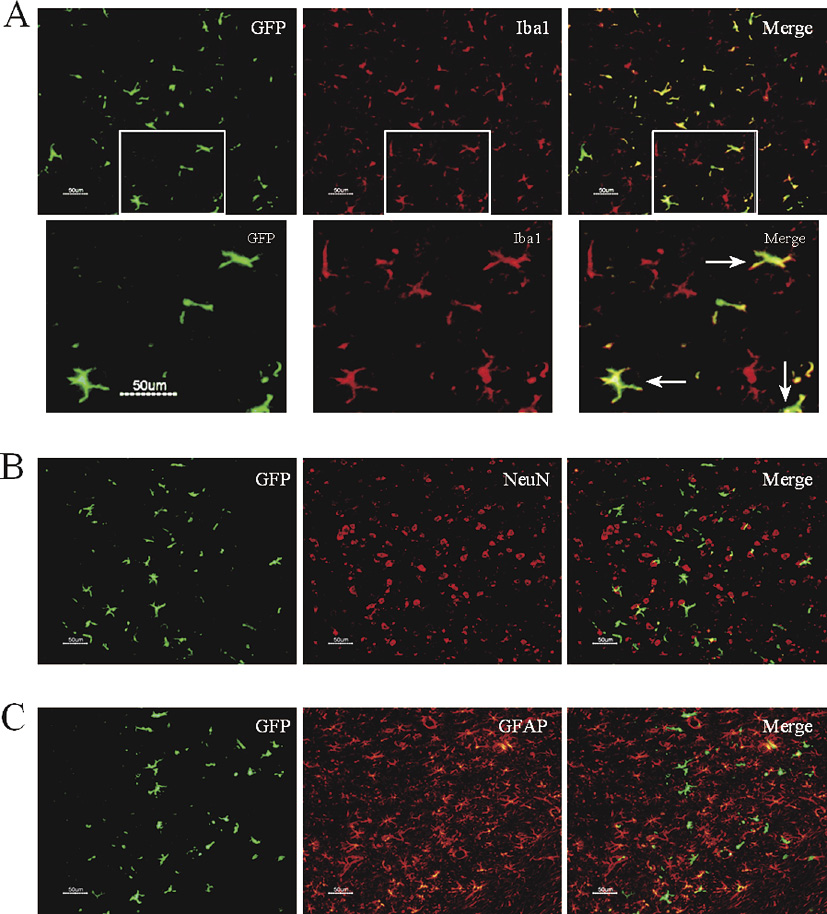
Phenotype identification of GFP+ cells in mouse brain by immunofluorescence double staining. Brain sections of the fAβ42-injected side in JTT-treated group were selected. (A) Sections were stained with anti-GFP (green) and anti-Iba1 (red). The superimposing fluorescence image was shown as merge image. The lower three panels represented the high-power inserts of corresponding upper panels. The arrows show some merged cells with characteristic highly ramified and elongated morphological appearance with small cell bodies. (B) Sections were stained with anti-GFP (green) and anti-NeuN (red). The superimposing fluorescence image was shown as merge image. (C) Sections were stained with anti-GFP (green) and anti-GFAP (red). The superimposing fluorescence image was shown as merge image. Scale bar, 50 μm.
At maturity, many organs possess original cells capable of self-renewal and multilineage differentiation known as adult stem cells (Fuchs and Segre 2000; Weissman 2000). Neural stem cells are found in several regions of the mature brain and spinal cord, in which they give rise to astrocytes and oligodendrocytes (Gage 2000). Being influenced by site-specific molecular cues, stem cells confined to the subventricular and hippocampal subgranular zones can also produce new neurons. Current evidence suggests that neural stem cells do not manifest the ability to generate microglia, and microglia in the adult stage are derived from the proliferation of inherent microglia, or the bone marrow precursor cells (Lawson et al. 1992; Asahara and Isner 2002).
Many studies confirmed that BMSC could enter the CNS, and differentiate into functional microglia (Priller et al. 2001a, b; Vallieres and Sawchenko 2003; Simard and Rivest 2004; Massengale et al. 2005; Malm et al. 2010). Although some researchers thought that BMSC could enter the normal brain with intact BBB (Vallieres and Sawchenko 2003; Simard and Rivest 2004), they were more easily attracted to the regions involved in neurodegenerative diseases or other neurological disorders (Priller et al. 2001a, b, 2006; Vallieres and Sawchenko 2003; Villeneuve et al. 2005; Simard et al. 2006). They have described that BMDC entered the brain, gathered, and differentiated into ramified microglia. Although the precise mechanisms are not clear, it is possible that damaged neurons transmit signals to inherent microglia, which produce specific chemokines, resulting in the gathering of BMDM.
Many studies on AD have confirmed that microglia were readily attracted to amyloid deposits, reducing the amyloid deposits (Malm et al. 2005; Simard et al. 2006). Simard et al. (2006) demonstrated that BMDM were specifically attracted to amyloid plaques in vivo and phagocytosed Aβ. More than 90% of microglia around senile plaques were newly recruited, they but not resident microglia could phagocytose Aβ, and this might be a beneficial mechanism in restricting disease progression (Simard et al. 2006). Boissonneault et al. (2009) reported that M-CSF could stimulate BMDM, degrade Aβ and improve the cognitive decline. Interestingly, similar results were reported when administrating G-CSF to AD mice (Sanchez-Ramos et al. 2009). Recently, Li et al. (2011) found that stem cell factor plus G-CSF augmented BMDM and lead to long-term reduction of Aβ deposition in AD brain.
Compound Kampo formulation is characteristic in traditional medicine, and it has numerous unique efficacies. In compound formulation, the dose of various active components is lower than their single therapeutic dose. Moreover, new active components and new effects might be found in compound formulation, and this is just the superior of compound herbal medicine. Compound formulation has so many advantages, such as low dosage, good validity, low toxicity and few side effects. Among more than one hundred kinds of compound herbal medicine, JTT is accepted to stimulate immune functions by enhancing phagocytosis, cytokine production, and antibody production. JTT is usually used to treat patients who are debilitated by chronic illness or cancer. Our previous study found that JTT activated microglia and induced microglial proliferation and activation without an increase in nitric oxide production. Moreover, bone marrow-derived macrophages from JTT-administered mice showed enhanced phagocytosis of fAβ42 (Liu et al. 2008). Hara et al. (2010) demonstrated that oral JTT decreased Aβ burden in AD transgenic mice by activating bone marrow-derived macrophages. They speculated that bone marrow-derived macrophages crossed BBB, developed into microglia, and phagocytosed aggregated Aβ in senile plaques.
In the present study, first we made a chimeric mouse model by transplanting fresh bone marrow cells from EGFP transgenic mice in order to chase the exogenous cells in mouse brain. We investigated whether BMDC could enter the normal brain or not, in order to provide additional evidence to present studies. In addition, we examined whether JTT plays a role in the transition and differentiation of BMDC into the normal brain. The results suggested that BMDC could cross BBB and migrate into the normal brain parenchyma, but these green cells were only found in the cerebellum with the relatively weak BBB. This finding is consistent with the report by Vallieres and Sawchenko (2003). JTT did not promote the migration of BMDC in the normal brain, whereas JTT was revealed to increase CD11b+ cells in blood and Iba1+ microglia in hippocampus. These results provide evidence that JTT could promote the proliferation of monocyte-macrophage lineage cells, and this was consistent with our published result (Liu et al. 2008). Thus, JTT did promote the proliferation of resident microglia in the brain that has been considered the immune privilege organ. In this part, previous reports are controversial (Priller et al. 2001a, b, 2006; Vallieres and Sawchenko 2003; Simard and Rivest 2004; Villeneuve et al. 2005; Simard et al. 2006). We believe our findings contribute to understanding the essence.
The AD model was established by intrahippocampal injection of fAβ42, and we investigated whether JTT plays any role in the homing of BMDC to the lesion. So many GFP+ cells were found gathering not only in the injection pathway but also around the injected lesion, while some cells penetrated into the injected fAβ42. Most cells adopted ramified appearance while some were round-like, and there was no difference between experimental group and control group in the cell number. Moreover, in the injection pathway and around the injected DPBS, many GFP+ cells were found as well. These results illustrated that injection of fAβ42 could induce the migration of BMDC into brain, gathering and differentiation of BMDC. The BMDC that invaded into the lesion might play a role in diminishing the lesion. Furthermore, the results also showed that mechanical trauma could induce the homing of BMDC, and in this study the trauma influenced the effect of fAβ42 to some extent. Further investigation must be performed using amyloid precursor-Tg mice.
Although there was no difference between two groups in BMDC gathering around injected lesions, we surprisingly found that for the non-neighboring regions of injected fAβ42, JTT increased the number of BMDC markedly. This result suggests that under the condition of local BBB damage, JTT promotes the homing of BMDC to the brain widely.
What kind of cells were the GFP+ cells that entered the brain? The results of phenotype identification showed that majority of the GFP+ cells in the parenchyma adopted microglia phenotype (Iba1+), demonstrating that BMDC could differentiate into microglia in the brain instead of neurons or astrocytes, even in the damaged brain, and JTT could not induce their differentiation into neurons and astrocytes. This result was in line with some reports (Vallieres and Sawchenko 2003; Hess et al. 2004; Simard and Rivest 2004; Malm et al. 2005; Simard et al. 2006), but was contrary to those observations demonstrating BMDC could generate neurons and astrocytes (Zhao et al. 2002; Weimann et al. 2003). Vallieres and Sawchenko (2003) thought that the discrepancy could be explained primarily by the use of unspecific antibodies, less sensitive histochemical methods, or the lack of high-resolution confocal analyses. In our point of view, unfractionated bone marrow contained at least two populations of stem cells: hematopoietic stem cells and mesenchymal stromal cells. To date, most studies showing differentiation of bone marrow cells into neurons and astrocytes used cultured mesenchymal stromal cells and most experiments were in vitro. We thought it was hematopoietic stem cells or myeloid progenitor cells that differentiated into microglia as Hess et al. (2004) suggested, although in this experiment we used whole bone marrow extracts instead of purified or clonally derived stem cells.
In summary, this study demonstrated that Kampo formulation JTT could induce microglial proliferation in normal brain. In AD brain, JTT could induce the homing of BMDC, which then differentiated into microglia, penetrated into the fAβ42-injected site, and phagocytosed it. This effect might reduce the disease, as well as playing an immune surveillance role in non-pathological brain regions. These findings suggest that the effect of JTT on BMDC might be a potential therapeutic strategy for AD. Further intensive investigations should be performed in larger animal groups to elucidate the underline mechanism in detail.
This study was partly supported by Grant-in-Aid for Scientific Research on Priority Area (17025056) and by a grant of Strategic Research Foundation Grant-aided Project for Private Universities from the Ministry of Education, Culture, Sports, Science and Technology in Japan. Huayan Liu is a fellow supported by Sasakawa Foundation. We thank Sasakawa Memorial Health Foundation and Japan China Medical Association for their kind support. We also thank Drs. Heii Arai and Nobutaka Hattori for their kind help.
We declare no conflict of interest.